Site-Specific Alkene Hydromethylation via Protonolysis of Titanacyclobutanes
- 17/385,528
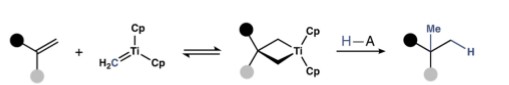
Methyl groups are ubiquitous in biologically active molecules. Thus, new tactics to introduce this alkyl fragment into polyfunctional structures are of significant interest. With this goal in mind, a direct method for the Markovnikov hydromethylation of alkenes was developed by FSU researchers. This method exploits the degenerate metathesis reaction between the titanium methylidene unveiled from Cp2Ti(µ-Cl)(µ-CH2)AlMe2 (Tebbe’s reagent) and unactivated alkenes. Protonolysis of the resulting titanacyclobutanes in situ effects hydromethylation in a chemo-, regio-, and site-selective manner. The broad utility of this method is demonstrated across a series of mono and di-substituted alkenes containing pendant alcohols, ethers, amides, carbamates, and basic amines.
Key Words : Chemical Synthesis