The Soret Effect in Polymer-Electrolyte-Based Electrochemical Cells
- US Patent Pending
The Soret effect arises when a temperature gradient is imposed on a multi-component system, inducing a concentration gradient. There is no comprehensive theory of the Soret effect that applies to all the systems that have been studied. Polymer electrolytes are a novel and interesting system in which to study the Soret effect due to the dissimilar properties of polymers and salts.
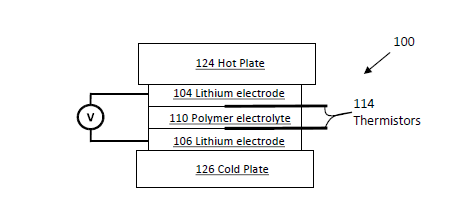
Polymer electrolytes provide a system in which the mobility of the components are dramatically different and in which the species solvating the ions (polymer segments) cannot transfer with the ion. This can lead to large partial molal free energy of transfer for ions in polymer electrolyte. In addition, the solid nature of polymer electrolytes precludes convection, which is a vexing source of error in thermal diffusion studies. Studies on polymer blends have found unexpectedly large Soret coefficients near a phase transition. With complex phase diagrams, polymer electrolytes provide an avenue to study this phenomena. In addition, the Soret effect in dry polymer electrolytes could potentially be used to convert waste heat into electricity and improve the efficiency of electrochemical cells.
This technology describes measuring the Soret coefficient in a dry polymer electrolyte by determining the concentration gradient that develops in an imposed temperature gradient. The concentration gradient may be determined using various methods including an electrochemical approach and by magnetic resonance imaging. Transient studies may be used to determine the thermal diffusion coefficient, providing another way to calculate the Soret coefficient. Consideration is given to higher order effects such as non-constant transport parameters by determining the temperature dependence of both thermal mass diffusion and thermal energy diffusion.