- Research Offices
- OHSP
- Clinical Trials
Clinical Trials
IRB-related guidance, information, instructions and other resources for the FSU research community

This Clinical Trials page provides the FSU research community with information about when a study may constitute a "clinical trial", researchers' additional responsibilities when planning or conducting clinical trials, and access to pertinent resources and other guidance. The information provided here is primarily focused upon matters with which the IRB has a special interest (i.e., ensuring that clinical trials conform to clinical trial-related human research regulatory requirements). Important note: federal funding agencies, other sponsors as well as regulatory agencies such as the FDA have additional requirements not specifically addressed here; questions and the need for information about those requirements should accordingly be redirected there. This page covers the following topics and more:
- When is a Study a Clinical Trial?
- What Do Researchers Need to Do?
- Sources of Regulatory Information
- Finding Related Resources
A subset of human research, clinical trials are subject to additional requirements imposed by different federal laws as well as study sponsors and publishers, due in part to the following: the use of regulated products such as drugs and devices; laws that mandate public access to information about clinical trials; heightened risks that are often associated with health-related interventions; and the involvement of study participants who may be vulnerable because of their poor health status and other factors. Knowing when your human research is a clinical trial for which additional requirements will apply may help to ensure appropriate IRB review of your studies, protection of study participants and ultimately compliance with applicable laws. Check out the panels below for more information.
For purposes of determining whether a study constitutes a "clinical trial" and may therefore be subject to clinical-trial related legal or other requirements, a threshold matter is ascertaining how a clinical trial is defined by the federal department or agency that may regulate or fund the study. Below are the definitions provided by the U.S. Food and Drug Administration (FDA), National Institutes of Health (NIH), and under the Federal Policy for the Protection of Human Subjects (aka the "Common Rule") that many federal departments and agencies have adopted.
- U.S. Food and Drug Administration (FDA) Definition of Applicable Clinical Trials (42 CFR Part 11; ClinicalTrials.gov)
Applicable clinical trials generally include:-
Controlled clinical investigations (other than phase 1 investigations) of any FDA-regulated drug or biological product for any disease or condition
-
Certain studies of FDA-regulated medical devices, excluding small clinical trials to determine feasibility and certain clinical trials to test prototype devices, but including FDA-required pediatric post-market surveillances of a device product
-
Interventional studies (with one or more arms) of FDA-regulated drug, biological, or device products that meet one of the following conditions:
-
The trial has one or more sites in the United States
-
The trial is conducted under an FDA investigational new drug application or investigational device exemption
-
The trial involves a drug, biological, or device product that is manufactured in the United States or its territories and is exported for research
-
-
- National Institutes of Health (NIH) Definition of Clinical Trial (NIH Policy)
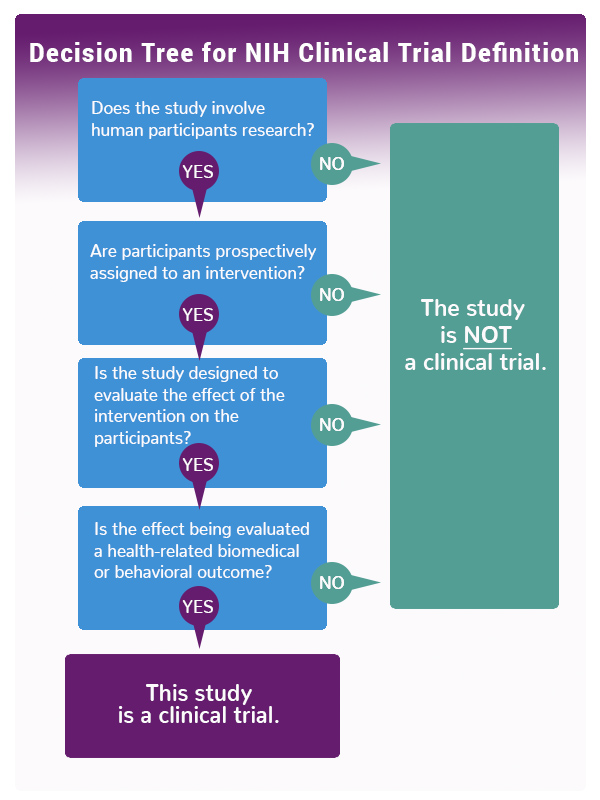
A clinical trial is a research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes. If the answer is “yes” to all four of the following questions, then the research study meets the NIH definition of a clinical trial and must be registered:- Does the study involve human participants?
- Are the participants prospectively assigned to an intervention (placebo or control)?
- Is the study designed to evaluate the effect of the intervention on the participants?
- Is the effect being evaluated a health-related biomedical or behavioral outcome?
See the infographic below or use this NIH Decision Tool to see whether your study falls within NIH's definition of the term clinical trial.
-
Federal Policy for the Protection of Human Subjects (45 CFR Part 46, section 46.102(b))
A clinical trial means a research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of the interventions on biomedical or behavioral health-related outcomes.
-
- Note that this definition may apply to studies that may or may not be subject to FDA regulations or funded by NIH, and which may be conducted or funded by other federal departments and agencies, non-federal sponsors and a wide range of research and academic institutions and organizations.
Infographic: NIH Decision Tree for Definition of a Clinical Trial
If your study is a clinical trial, then you are responsible in accordance with federal law and FSU policy for compliance with the following additional requirements, as may be applicable.
1. Data and Safety Monitoring Plan. All clinical trial studies must include a Data and Safety Monitoring Plan (DSMP) that will provide appropriate oversight and monitoring, which will be evaluated by the IRB for sufficiency. Click on the panel below to review DSMP requirements.
DSMPs may--owing to differences among clinical trial studies--vary greatly from another, but the level of monitoring must be commensurate with risks and with the size and complexity of the trials. Refer to section 18 of the FSU approved protocol templates; templates are available in RAMP IRB, under the IRB, Library and Templates tabs. Refer also to the NIH Data and Safety Monitoring page for policy and related guidance; requirements may differ among the NIH and its Institutes and Centers, so plan accordingly if your study is so funded. For clinical trials that are not federally funded, DSMPs should still include elements required by NIH. As well, study sponsors may include DSMPs into the protocol, but for other clinical trials the study team is responsible for developing and implementing the DSMP.
DSMPs by Clinical Trial Type*:
Generally, for Phase I, “first in human” or post-approval clinical trials, a DSMP may include continuous data and safety monitoring by the study team of study participant safety, with frequent reporting to federal agency or sponsor staff as well as reporting of any incidents involving participant health and safety to the IRB. An independent safety monitor or Data and Safety Monitoring Board (DSMB) is generally not required, but may be appropriate if the clinical trial has multiple clinical sites, is blinded (masked), involves treatment delay or medication washouts or placebo controlled studies in clinical populations, or involves high-risk interventions or vulnerable populations.
For Phase II clinical trials, data and safety monitoring may be similar to that of a Phase I trial, to include study team monitoring supplemented by other individuals outside of the study team who have expertise that is relevant to the study and who assist the study team with interpreting the data to ensure patient safety. Again, an independent safety monitor or a DSMB may be appropriate if the clinical trial has multiple clinical sites, is blinded (masked), involves treatment delay or medication washouts or placebo controlled studies in clinical populations, Investigational New Drug (IND)/Investigational Device Exemption (IDE) studies, or involves high-risk interventions or vulnerable populations.
For Phase III clinical trials, a Data and Safety Monitoring Board (DSMB) is required; the DSMB must be composed of experts relevant to the study, who regularly assess the clinical trial and offer recommendations to federal agencies, sponsors and the IRB regarding the clinical trial’s continuation.
*Phases of Clinical Trials
Clinical trials are conducted in “phases", each with a different purpose for answering different research questions:
Phase I trials — An experimental drug or treatment in a small group of people (generally 20–80) for the first time. The purpose is to evaluate a drug's metabolism and pharmacologic actions in humans, the drug's safety and identify side effects. This phase may be similar to a pilot stage for feasibility testing of medical devices.
Phase II trials — The experimental drug or treatment is administered to a larger group of people (generally 100–300) to determine its effectiveness for a particular indication for patients with a disease or condition, and to further evaluate its safety, including short-term side effects and risks. This phase may be similar to the pivotal stage to examine the safety and effectiveness of medical devices.
Phase III trials — The experimental drug or treatment is administered to large groups of people (generally 300-3,000) to obtain additional information to confirm its effectiveness and safety, monitor side effects, compare it with standard or equivalent treatments and provide a basis for labeling.
Phase IV trials — After a drug is licensed and approved by the FDA researchers track its safety, seeking more information about its risks, benefits, and optimal use. This is similar to a post-market stage for medical devices, and seeks answers to the question, "what else needs to be known?" such as long-term safety, efficacy and optimal use.
See NIH Glossary of Common Terms [link]; see also NIH Policy for Data and Safety Monitoring ( June 10, 1998); Further Guidance on a Data and Safety Monitoring for Phase I and Phase II Trials (June 5, 2000; NOTICE: OD-00-038); and 21 CFR 312.21 (Phases of an investigation).
2. Informed Consent. All clinical trials must include an informed consent process. Click on the panel below to review informed consent requirements.
The informed consent process, the consent forms used, and documentation of consent must adhere to the following:
- Every clinical trial must include an informed consent process; there is no exception.
- The informed consent of individuals must be obtained and documented before the clinical trial may be initiated. No waiver of consent or documentation of consent will be granted for ANY clinical trial, except for certain emergency research in accordance with 61 Federal Register 51531 (1996) or Title 21 of the U.S. Code of Federal Regulations Part 50, section 50.24 [link]. Even these exceptions include an informed consent process!
- Informed consent must include the following statements:
- Whether participants or their insurance may be charged or billed for any clinical trial-related services, items or other expenses, and if participants may incur any deductibles, co-pays, co-insurance or full pay. Note that the OHSP/IRB does not otherwise provide researchers with review, approval, technical support or other resources about clinical trial-related budgeting, billing or reimbursement; consult the study sponsor for this information.
- That any clinical care or services that participants may be provided during the time of their clinical trial participation may be charged or billed to participants or their insurance, and that as a result participants may incur deductibles, co-pays, co-insurance or full pay.
- A description about how participants may be referred for necessary medical care in the event of any clinical trial research-related injury, and whether and/or how such participants will be compensated for clinical trial-related injuries. If participants will not be compensated for clinical trial-related injuries, this must be clearly stated.
- ClinicalTrials.gov registration.* If subject to FDA requirements, the consent form must include the following specific and verbatim statement about ClinicalTrials.gov registration: "A description of this clinical trial will be available on http://www.ClinicalTrials.gov, as required by U.S. Law. This Web site will not include information that can identify you. At most, the Web site will include a summary of the results. You can search this Web site at any time." Note that the above wording cannot be changed. The applicable FSU approved consent form includes this template language. Consent templates are available in RAMP IRB, under the IRB, Library and Templates tabs. For a brief overview about ClinicalTrials.gov and registration requirements, see our About ClinicalTrials.gov panel below.
- Publicly post the consent form.* For any study that is supported by federal department or agency funding and for any applicable clinical trial under FDA regulations, an unsigned copy of the study consent form that was approved by the IRB must be posted to a publicly available federal website after the research has been closed to recruitment and no later than 60 days after the last study visit of any subject.
*Note that the OHSP/IRB does not provide support to researchers for their use, navigation or problems in using ClinicalTrials.gov, and will not remind study teams to publicly post their unsigned copies of IRB approved consent forms; the OHSP/IRB does not track when clinical trials are closed to recruitment or when 60 days since a subject's last study visit have passed.
3. Other FDA requirements. Click on the panel below to see more about other FDA requirements.
Besides the above clinical trial-related requirements pertaining to informed consent content, posting and ClinicalTrials.gov registration, many additional FDA requirements and regulations may apply to clinical trials that involve drugs, devices, biologics, nutritional supplements, and combination products. These may require that researchers submit special Investigational New Drug (IND) or Investigational Device Exemption (IDE) applications or obtain other documentation of FDA approval or clearance for use of FDA-regulated products before submitting the clinical trial study to the FSU IRB for review.
Visit our Decision Trees web page and scroll down to the FDA-regulated Products and Test Articles section to review our draft FDA drug and device algorithms to learn more about FDA requirements and restrictions on the use in research or clinical trials of certain products or test articles such as drugs, biologics, devices and dietary supplements.
The IRB will require documentation that FDA requirements have been satisfied before granting approval. See section 5 of the FSU approved template protocol, HRP-503 - TEMPLATE PROTOCOL.
To access FDA information about clinical trial-related human subject protection requirements, including guidance, compliance and enforcement activities, Good Clinical Practice educational materials, and International Conference on Harmonization (ICH) documents, visit the FDA's Clinical Trials and Human Subject Protection page [link].
4. Training. Click on the panel below to find out about training requirements.
Study team members involved in conducting clinical trial studies that are federally funded or subject to FDA regulations are required to complete certain FSU training. This includes completion of initial and refresher CITI training in human subjects research, and completion of Good Clinical Practice (GCP) training. Learn more about the the GCP training requirements at the NIH [link] and FDA [link].
Visit our CITI Training web page to find out how to access the required CITI training modules for human subjects research and GCP; these are separate courses.
ClinicalTrials.gov is a web-based, publicly available resource about federally and privately supported clinical trials conducted in the United States and abroad, both current and past studies. This resource is intended to provide the public with ready access to information about clinical studies involving many different diseases and other health conditions. Researchers' registration and/or posting of certain clinical trial information to the web site is required if their study is funded by the NIH or another federal agency and/or subject to FDA regulation.
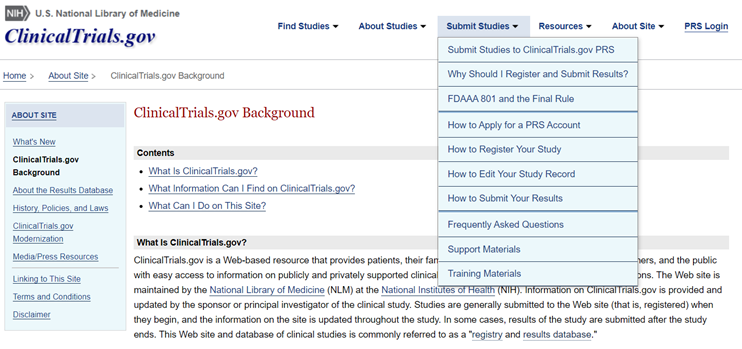
Clinical trial study information will include descriptions of the studies, copies of IRB-approved consent forms, study summary results and adverse event information, as may be applicable, for completed studies. The site requires that researchers and their institutions comply with certain requirements, such as updates and correcting errors. To learn more about ClinicalTrials.gov-related requirements for clinical trials studies, including background, the underlying laws, how to register studies, submit results, obtain training or FAQs, visit the ClinicalTrials.gov main web site or the ClinicalTrials.gov background page. A screenshot of the site is provided below.
Important Note! The OHSP/IRB does not provide support to researchers for their use, navigation or problems in using ClinicalTrials.gov. For FSU administrative assistance with ClinicalTrials.gov-related requirements, contact the FSU Office for Clinical Research Advancement (OCRA). [link]
FAQs for ClinicalTrials.gov: Visit this page at https://clinicaltrials.gov/ct2/manage-recs/faq to obtain information and instructions for using the National Library of Medicine's ClinicalTrials.gov site. Numerous topics are covered, including how to register, deadlines, information and documents to submit, penalties for non-compliance and contacts. A snapshot of the site's background page and "Submit Studies" tab is depicted below.
 |
Below are listed some key, authoritative sources of information related to clinical trial requirements:
- National Institutes of Health (NIH): https://grants.nih.gov/policy/clinical-trials.htm (NIH web page for grantees and contractors, covering clinical trial requirements; information includes definitions, funding opportunities, templates, informed consent posting requirements, and training)
- National Institute of Allery and Infectious Diseases (NIAID/NIH): https://clinregs.niaid.nih.gov/ (NIAID's online database of many country-specific clinical research regulatory information)
- National Library of Medicine (NLM): https://clinicaltrials.gov/ct2/home (NLM's ClinicalTrials.gov home page, at which site the public may access information about clinical trials and researchers may register their clinical trial studies; for researchers, click on the "Submit Studies" tab)
- National Library of Medicine (NLM): https://clinicaltrials.gov/ct2/manage-recs/faq (FAQs covering numerous topics including how to register, deadlines, information and documents to submit, penalties for non-compliance and contacts)
- U.S. Food and Drug Administration (FDA) (for researchers): https://www.fda.gov/science-research/science-and-research-special-topics/clinical-trials-and-human-subject-protection (FDA web page providing researchers with information about clinical trials, including guidance, compliance and enforcement information, Good Clinical Practice educational materials, and contact information)
- See also FDA Guidance for Clinical Trial Sponsors (also applicable to investigator-sponsors): Establishment and Operation of Clinical Trial Data Monitoring Committees: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/establishment-and-operation-clinical-trial-data-monitoring-committees
- U.S. Food and Drug Administration (FDA): https://www.fda.gov/regulatory-information/search-fda-guidance-documents/e6r2-good-clinical-practice-integrated-addendum-ich-e6r1 (FDA Guidance Document: Good Clinical Practice: Integrated Addendum to ICH E6(R1); provides detailed information, instructions and guidance for conducting clinical trials, including researchers' qualifications, preparing a clinical trial protocol, informed consent, safety reporting and much more)
- U.S. Food and Drug Administration (FDA) (for patients): https://www.fda.gov/patients/clinical-trials-what-patients-need-know (FDA's web site intended to provide patients with clinical trial information, including distinguishing clinical trials from treatment, searching for clinical trials, and tutorials)
- European Medicines Agency (EMA): https://www.ema.europa.eu/en/human-regulatory/research-development/clinical-trials-human-medicines (EMA's Clinical Trials page, with information about data submission, safety reporting, clinical trial regulation, training and reference materials)
- International Committee of Medical Journal Editors (ICMJE): https://www.icmje.org/ (the ICMJE recommends that all medical journal editors require registration of clinical trials, including at ClinicalTrials.gov or the World Health Organization's clinical trials registry; use the Quick Links for Clinical Trial Registration. Researchers who plan to submit manuscripts for publication are advised to contact their publishers to confirm the need for such registration)
- World Health Organization (WHO): https://www.who.int/clinical-trials-registry-platform (WHO's Clinical Trials Registry platform, providing information about the platform, including searching for clinical trials and related findings, international standards for clinical trial registries, and how to register a clinical trial)
- Office of the Inspector General (OIG), U.S. Department of Health and Human Services (DHHS): https://oig.hhs.gov/ (among OIG-audited NIH-funded clinical trials, OIG found that over half were not compliant with federal clinical trial reporting requirements. Both the OIG and NIH concur with the need for additional enforcement actions for non-compliance. Access the report)
Listed below are select organizations (or the organizations' clinical trial-related pages) that provide the research community with clinical trial-related resources and information, including topics and matters such as clinical trial staffing, insurance coverage, workload, contract negotiations, site assessments and clinical trial professional support. Others will be added as they become available. Cut and paste the URL/link into your web browser.
- American Society of Clinical Oncology (URL: https://www.asco.org/research-data/clinical-trials/clinical-trial-resources)
- Society of Clinical Research Associates (SOCRA) (URL: https://www.socra.org/)
- Society for Clinical Trials (URL: https://www.sctweb.org/)
- Southwest Oncology Cancer Research Network (URL: https://www.swog.org/clinical-trials/clinical-research-resources)
- U.S. Veterans Administration, Office of Research Development (URL: https://www.research.va.gov/resources/ORD_Admin/clinical_trials/)
- WCG CenterWatch (URL: https://www.centerwatch.com/)
For materials that clinical researchers may find useful* (articles; DSMP checklists, budgeting and guidelines; DSMB charters, templates and confidentiality; data sharing; manual of procedures; and study administrative forms), check out the following:
- Clinical Trial Registry Errors Undermine Transparency, The Scientist, August 2, 2022 (Email us for a copy for educational use; FSU employees only)
- Principles of Designing a Clinical Trial: Optimizing Chances of Trial Success, El-Hagrassy et al., Current Behavioral Neuroscience Reports 5, 143-152 (2018) (DHHS Public Access, PMID: 30467533) [link]
- Georgetown University's Clinical Research Operations Office: https://clinicaltrials.georgetown.edu/investcoord/resources/
- National Institute on Aging's Clinical Research Study Investigator's Toolbox: https://www.nia.nih.gov/research/clinical-research-study-investigators-toolbox
- Emory University's Clinical Trial Resources: https://ctac.emory.edu/resources/trial-tools.html
- University of Florida Clinical and Translational Science Institute's Clinical Research Toolkit: https://www.ctsi.ufl.edu/research/research-support/irb-consults/clinical-research-toolkit/
*Note that where FSU OHSP/IRB templates are provided through RAMP IRB (under the IRB, Library and Templates tabs), that the FSU approved templates must be used.
|
OSHP and IRB Review Algorithm for Clinical Trials Use the Decision Trees link on the left to access our Clinical Trials/Human Research Workflows algorithm to get a quick overview of the FSU IRB human research review process for clinical trial studies. Key decision points are depicted, and explanatory notes provided.
|
|