- Research Offices
- OHSP
- FAQs

FREQUENTLY ASKED QUESTIONS & ISSUES
Below are listed some of the most frequently asked questions, covering General, RAMP IRB-related and Specific FAQs. To find an answer, click on an FAQ. To return to the list, click again on the FAQ or "v" symbol. For FAQs specific to student-led research, visit this student-led research FAQs page.
Search FSU
- An IRB is a committee composed of scientists and non-scientists (e.g., professionals as well as laypersons) that may be affiliated with an institution or organization, as well as persons not affiliated with these institutions or organizations (referred to as IRB members), that review and provide ethical and regulatory oversight of human research that is proposed and conducted by other persons affiliated with those institutions or organizations. IRB members are expected to have the background, experience and expertise required to ascertain the ethical and regulatory acceptability of proposed and ongoing research, primarily to help ensure the protection of the rights and welfare of individuals who serve as human research participants (human subjects) in research.
- The Florida State University (FSU) has an IRB. Institutions like FSU which receive federal funds to conduct human research are required by federal law to have an IRB. Federal regulations provide the FSU IRB with the authority to approve, require modifications in (to secure approval), or disapprove all research activities covered applicable law and FSU policy. Furthermore, while research that has been approved by the FSU IRB may be subject to further review and approval or disapproval by FSU officials, these officials may not approve the research if it has not been approved by the FSU IRB.
- The IRB is not permitted by law to retroactively review human research.
- Only human research that complies with applicable law and policy is approved by the FSU IRB.
- Proposed human research that does not comply with applicable law and/or policy or the IRB’s requirements will be returned to the study team until the proposed research meets the IRB's criteria and conditions for approval.
- Previously approved research which is determined to violate applicable law and policy and the IRB conditions for approval will be suspended and may be terminated. Violations may require reporting to FSU officials, federal regulatory agencies and study sponsors. Subsequent proposed and any on-going research conducted by the study team may be subject to heightened review scrutiny and restrictions.
- Federal law also requires institutions such as FSU to establish and follow policies that implement requirements for human research regulatory review. At FSU, the overarching policy is 7-IRB-0, and is accompanied by subordinate procedures (standard operating procedures) that are maintained by the FSU Office for Human Subjects Protection (OHSP) in the RAMP IRB Library.
- Federal law and policy does NOT permit IRB or other human research regulatory review or approval to be performed or granted retroactively (after you have initiated human research). Plan to prepare and submit your research well in advance.
- IRB review may follow one of several or more pathways. Like the federal law that requires FSU to have an IRB to review human research, the requirement that an activity actually undergo a particular pathway of IRB or other human research regulatory review before the activity is permitted to begin is also governed by federal law. The law dictates the conditions, such as review of research risks, under which an activity must undergo such review as well as how review may be performed.
- Depending upon the nature of the activity, research may require complete IRB review: through a monthly convened meeting of the IRB; through an expedited or IRB subcommittee process; or by way of administrative review that may be performed by OHSP staff, some of whom also serve as IRB members. Check out our Human Research Review web page for more details about these different types of review processes, including the anticipated review turn-around time.
- IRB review is scheduled only after proposed human research is deemed by the OHSP as "review ready" (includes the required information and materials submitted in RAMP IRB).
- Save yourself time and effort! Carefully look over our HRP-308 Worksheet - Pre-Review for the list of information and materials that OHSP will check in order to deem research as IRB "review ready". Submissions that are missing information and materials will be returned to the study team for correction. HRP-308 is accessible in RAMP IRB, under the IRB, Library and Worksheets tabs (scroll down the HRP-308).
- To conform to the federal legal requirement to maintain IRB records of communications with researchers, we do not provide regulatory determinations via email or telephone.
- In order to receive a determination, complete the HRP-503d - Template Determination Form and submit this form in RAMP IRB.
- Once RAMP, under the IRB tab, click “Create New Study” to complete the RAMP IRB application (follow the IRB Researcher's Guide beginning on page 6 for detailed instructions). Then upload your completed determination form under question #8 in lieu of your protocol. Click “Submit” on your study workspace and your submission will be routed to us for review. We will get back to you with a response.
- Note a distinction between activities that are not research as that term is defined (for which an OHSP administrative, not an IRB, determination may be made) and activities that are deemed exempt from further IRB review (for which either an administrative or an IRB determination is required. At FSU, only the OHSP or IRB is permitted to officially determine whether an activity satisfies federal regulatory criteria for OHSP or IRB review.
- Any researcher, faculty, staff, student, contractor, volunteer or other person affiliated with the University who plans to conduct research involving the collection or use of an individual's information by (1) interacting or intervening with the individual or (2) obtaining the individual's information from another source, will require IRB review and approval or regulatory clearance before the research is undertaken. Federal law does not permit retroactive review; human research undertaken before review and approval or clearance is considered a violation of federal law.
- All requests for review must in accordance with University policy 7-IRB-0 be submitted in the University's Research Administration Management Portal (RAMP) Institutional Review Board (IRB) online electronic protocol management system ("RAMP IRB").
- As a quick reference and if you are interested in knowing about how research and creative activities are deemed to require OHSP and/or IRB review, click on this Decision Trees link (also on the left) and scroll down to the Engagement algorithm (in "What activities require IRB review?"). The chart provides a high-level overview of key decision points.
- If your research will NOT involve human subjects as depicted in the Engagement algorithm, then no IRB review is generally required. IMPORTANT NOTE: For (1) researchers who may plan to publish or pursue other scholarly dissemination of their research (owing to journal or related requirements), (2) students who may use their research in their dissertations and theses (due to manuscript clearance office requirements), or (3) those applying for federal funding (applicable grant and contract regulations), official IRB approval or clearance documentation specific to a study may still be required. Consult the journal, manuscript clearance office, sponsor or other relevant offices for their requirements and plan accordingly. If your research will not involve human subjects BUT any of above 3 or similar circumstances apply to your activity, then before your research is undertaken, create a submission in RAMP IRB and complete and submit our HRP-503d determination form (in lieu of a study protocol) and any attachments (variables list from prior studies data) to request review; the HRP-503d Template Determination form is accessible by logging into RAMP IRB, and navigating to the IRB, Library and Templates tabs.
- If there is a question about whether an activity requires IRB review, submit in RAMP IRB a completed HRP-503d - Template Determination Form and related materials; OHSP will review the form and related materials and let you know whether further IRB review is required and if so, what additional information and materials must be submitted. The HRP-503d Template Determination form is accessible by logging into RAMP IRB, and navigating to the IRB, Library and Templates tabs. See this Request a Determination video tutorial to see how to submit the HRP-503d form in RAMP IRB.
Alternatively, Contact us with this question but submission of a completed HRP-503d - Template Determination Form and related materials may save time and effort as well as answer many questions in advance.
- The following activities do not usually require OHSP or IRB review (these are examples only; carefully review the criteria): Oral history, journalism, biography, literary criticism, legal research, and historical scholarship, including the collection and use of information that focus directly on the specific individuals about whom the information is collected AND from which activity or analyses of the collected information there is no intent to draw conclusions or generalize findings beyond the specific individuals involved in the activity. However, if the activity or analyses of collected information will be used to document and draw conclusions about the individuals’ collective experiences, inform policy or otherwise generalize findings beyond the specific individuals involved, then these activities will require OHSP or IRB review.
- If your activity is atypical or if you are not sure if your activity will require OHSP or IRB review, submit in RAMP IRB a completed HRP-503d - Template Determination Form and related materials; OHSP will review the form and related materials and let you know whether further IRB review is required and if so, what additional information and materials must be submitted. The HRP-503d Template Determination form is accessible by logging into RAMP IRB, and navigating to the IRB, Library and Templates tabs. See this Request a Determination video tutorial to see how to submit the HRP-503d form in RAMP IRB.
Generally no. Oral History research projects, in general, are not designed to contribute to generalizable knowledge and do not involve "research" as defined by DHHS at 45 CFR 46.102(d), and therefore do not need to be reviewed by the IRB. Oral History activities are usually intended to create a record of specific historical events and, as such, are not intended to contribute to generalizable knowledge.
General Principles for evaluating Oral History activities:
- Oral history activities, such as open ended interviews, that ONLY document a specific historical event or the experiences of individuals without an intent to draw conclusions or generalizable findings would NOT constitute "research." Example: An oral history video recording of interviews with holocaust survivors created for viewing in the Holocaust Museum. The creation of the video does not intend to draw conclusions, inform policy, or generalize findings. The sole purpose is to create a historical record of specific personal events and experiences related to the Holocaust and provide a venue for Holocaust survivors to tell their stories.
- Systematic investigations involving open-ended interviews that are designed to develop or contribute to generalizable knowledge (designed to draw conclusions, inform policy, or generalize findings) would constitute "research" as defined by federal regulations. Example: An open ended interview of surviving Gulf War veterans to document their experiences and to draw conclusions about their experiences, inform policy, or generalize findings.
- Oral historians and qualitative investigators may want to create archives for the purpose of providing a resource for others to do research. Since the intent of the archive is to create a repository of information for other investigators to conduct research as defined by 45 CFR part 46, the creation of such an archive for such purpose WOULD constitute research under 45 CFR 46. Note however that such research may constitute exempt research; for information about the exempt research review pathway, visit our Human Research Review page, scroll down to the panel "IRB Review Pathways: New or Initial Studies" and refer to Exempt Review.
Example: Open ended interviews are conducted with surviving Negro League Baseball players in order to create an archive for future research. The creation of such an archive would constitute research under 45 CFR 46 since the intent is to collect data for future research.
Other activities involving open ended, qualitative interviews that have similar characteristics can involve research as defined by the federal regulations, when the activities are part of a systematic investigation designed to develop or contribute to generalizable knowledge.
Note: Even though a specific oral history project may not fit the definition of "research" under the federal regulations, the research must still be conducted in accordance with ethical and legal standards appropriate to oral history, including consent, and legal releases as appropriate. FSU encourages investigators to visit the following Oral History Association links for more information regarding principles and best practices.
Sometimes, especially if the activity is not precisely delineated or under other limited circumstances. Generally however, an activity that only and unequivocally constitutes QI does not require IRB review, unless the QI activity may involve any greater risk than is ordinarily associated with the care, services, practices, processes or outcomes being evaluated—if so, then IRB review is required. Also, an activity that involves both QI and human research will in accordance with applicable human research regulations require regulatory or administrative review in order to determine the need for and type of IRB review, if any.
- The key difference for purposes of determining whether IRB review may be required is that QI is intended solely to improve the way in which an institution’s care or services are provided to the institution’s or formal consortium of institutions’ patients, residents, clients, beneficiaries or other stakeholders who are directly affected by the QI project. That is, QI findings or outcomes are intended to only apply to those processes and structures directly involved in the QI project. In contrast, research is defined under federal law as a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge; findings or outcomes are primarily intended to apply to other contexts and individuals beyond those directly affected by or involved the research. Keep in mind that when QI project findings or outcomes are also intended to be applied to other contexts or individuals, then IRB review is required. Use our “Quality Improvement or Research” algorithm for further guidance.
- Except as indicated above, a QI activity does not need to be submitted for IRB review in accordance with applicable human research regulations. However, for many professional and scholarly publication purposes as well as for graduate office graduation/manuscript clearance requirements, an official human research/IRB regulatory determination may be required. If so, then you must submit your activity for regulatory or administrative review BEFORE UNDERTAKING YOUR ACTIVITY; applicable law does not permit retroactive review or determinations once such activities have commenced.
- Before you plan a project that may involve QI, check out and carefully review the “Quality Improvement or Research” algorithm, and design your project accordingly. Importantly, be sure that you explicitly describe in detail your intent, make certain that the description of your activities is consistent with your intent, and that you have sufficiently addressed any matter for which a distinction between QI and research can be unequivocally made. Contact OHSP if necessary. While not dispositive for purposes of a formal determination (for which only the OHSP or IRB have regulatory authority), the algorithm may serve as a quick reference and save you time and effort.
- A range of different documents and materials that will or should be used to plan and conduct your study must be submitted in RAMP IRB for review. For a handy checklist of required documents and materials, refer to section "Checklist of Information to Attach" towards the end of the "IRB Researcher's Guide", which guide is accessible in the RAMP IRB Help Center [link]. For many of those documents, use of our FSU approved templates is required.
- To see what key materials and information must generally be provided for all submissions, be sure to also click on the ? button in the following RAMP IRB sections: Basic Study Information--Item 8 (Attach the protocol); Study-Related Documents--Items 1-3 (Consent, Recruitment materials, and Other attachments); and Local Site Documents--Items 1-3 (Consent, Recruitment materials, and Other attachments). Submissions that fail to include these key materials and information or which fail to conform to these requirements or templates will be returned for correction.
- All templates are available in RAMP IRB under the IRB, Library and Templates. Follow templates instructions carefully, as these are designed to provide the IRB with information that is necessary for the IRB to ascertain the acceptability of the research based upon federal regulatory criteria for approval. Save time and effort: submissions that do not use or follow the templates will be returned for correction.
- Common documents and materials include, for example, the study protocol; actual measures and instruments and anything that human subjects will be asked to read, complete, watch or use; recruitment fliers and messages; consent forms; and outside approvals. Other materials may also be required depending upon study parameters and activities.
- The RAMP IRB SmartForms will include instructions and directions for you to follow for submitting and uploading study-related information, documents and materials. You are required to submit the complete study protocol (describing, e.g., the study’s background, rationale, objectives, design, sample, methodology, statistical considerations, organization and settings, sites or locations).
- Studies that involve human subjects (obtaining their data or involving interaction) generally require a consent process through which you will recruit and ask prospective subjects for their permission to involve them in your study.
- The OHSP and IRB provide a wide selection of protocol and consent templates for your use; these templates are tailored to different types of studies (biomedical; non-biomedical, such as social, behavioral or educational studies) and study populations (e.g., children, adults, limited English language proficiency). Use only the templates available in RAMP IRB under the IRB, Library and Templates tabs. OHSP and IRB criteria for review follow the templated protocols; if you do not use the templates your submission will be returned for correction.
- If you will only obtain secondary (previously collected) data but not have any interaction with human subjects, then you must provide a list of all data variables or data dictionary (highlighting or indicating what data variables will be obtained).
- If you will directly or indirectly interact with human subjects (surveys, interviews, focus groups, collect any other data or information, conducted in-person, face-to-face, virtually, remotely or by any other means), then you must provide ANY and ALL material that will be seen, heard, read or completed by or for a human subject, including recruitment fliers or messages, videos, images, and data collection instruments and measures. Sample measures or links to materials do not suffice for IRB finalization.
- Copyrighted surveys, questionnaires, instruments and other measures: Surveys, questionnaires, instruments and other measures MUST be provided to the IRB for review, regardless of whether these are copyrighted or not. Use and/or submission of a copyrighted instrument for only IRB review may be considered a “fair use” of copyrighted work since such use is clearly intended for research purposes; use is limited to only the IRB in its regulatory review of research involving such copyrighted instrument; no further disclosure or use of the copyrighted instrument is made by the IRB except among the IRB or in communications with the study team specifically about the copyrighted instrument; and use of a copyrighted instrument by the IRB for its review is not intended nor likely to have any effect upon any potential market for the instrument. If you need to first purchase or obtain permission to use copyrighted instruments so that these may be provided to the IRB for review, then contact the copyright holder to purchase and/or obtain their permission. After IRB approval, you may then proceed as needed or directed by the copyright holder to further purchase and/or use the copyrighted instruments for other, non-IRB research purposes. Additional information about research “fair use” of copyrighted materials is found at this U.S. Copyright Office link: https://www.copyright.gov/fair-use/. To obtain assistance with requesting copyrighted materials or permissions, visit this FSU Libraries link: https://guides.lib.fsu.edu/copyright.
- Save yourself time and effort! Carefully look over our HRP-308 Worksheet - Pre-Review for the list of information and materials that OHSP will check in order to deem research as IRB "review ready". Submissions that are missing information and materials will be returned to the study team for correction. HRP-308 is accessible in RAMP IRB, under the IRB, Library and Worksheets tabs (scroll down the HRP-308).
- This will depend upon several key factors: if your study (1) is complete, is accompanied by required documents, and satisfactory responses have been provided to any requested clarifications; (2) formally documented by OHSP as exempt and not subject to complete IRB review; (3) will require IRB committee (at a scheduled monthly meeting) or non-committee (subcommittee, also referred to expedited, for which review may be done at any time) review; and (4) requires any other outside or ancillary review (e.g., another institution's IRB reviews; safety reviews for use of hazardous materials or radiation-emitting devices; ethics or conflicts of interest reviews) outside of the OHSP or IRB. With the above caveats, particularly item (1) above:
- Exempt studies may receive clearance within 2 weeks or 10 business days, often less;
- Non-committee reviews may be completed within 4 weeks;
- Committee reviews may be completed within 6 weeks;
- IF outside or ancillary reviews are required, this may add time before a study may begin, so plan accordingly, such as obtaining these reviews before or while your study undergoes IRB review.
- Review turn-around is also subject to other factors, such as IRB reviewer availability or workload, complex studies (e.g., studies involving use of drugs, medical devices and other products subject to additional federal laws; agreements or arrangements with other outside agencies, institutions or sponsors).
- Save yourself time and effort! Carefully look over our HRP-314 Checklist - Criteria for Approval for the list of federal regulatory criteria that the IRB must apply to their review of research before granting approval. Submissions that are missing information and materials necessary for the IRB to indicate criteria have been satisfied will be returned to the study team for correction. HRP-314 is accessible in RAMP IRB, under the IRB, Library and Checklists tabs.
- While ALL studies once approved require on-going oversight by the IRB, SOME studies (e.g., studies in data analysis only with no interactions with study subjects; studies officially determined by the IRB to involve only minimal risks to study subjects) do not require the formal and focused annual (or other more frequent) continuing review that is required of other studies. When this continuing review is not applicable, IRB approval letters indicate no expiration date. Refer to the FAQ about closing out a study when your study is actually complete so that the study may be officially closed to further IRB oversight and our records updated accordingly.
-
If you leave FSU, you must either close your study or (if the study is to remain on-going at FSU) modify your study to designate another FSU researcher as the study’s principal investigator (PI).
- To close your study in RAMP IRB, follow these steps: create and submit a study continuing review, complete the Continuing Review/Study Closure Information items as applicable (the first 4 research milestones must be checked), acknowledge that the study will be closed, discard any open follow-on submissions related to the study, complete the remaining items, attach any supporting documents as may be needed, then select Save, Finish and under Next Steps select Submit. Your Study Closure submission will move into the queue for review and further handling.
- To designate another FSU researcher as the study’s PI, follow these steps: create and submit a study modification, indicating the indicating the scope as “other parts of the study,” providing modification information (e.g., status of study enrollment), and the explain that you will designate a new FSU PI. On the Basic Study Information page, under Local Principal Investigator, indicate the new FSU PI, make any necessary changes to this or other pages, and answer Yes or No to the Additional Requirement page about the PI’s student status (and if Yes, indicate the name of the faculty advisor or major professor), then Finish and Submit (Under Next Steps) the modification. Your modification will (after faculty advisor review for a new FSU student PI, if applicable) move into the queue for review and further handling.
- Note that if another FSU researcher is designated as your study’s PI, you may remain on the study team provided that your new institution (if you have a new institution) is aware of your continuing role on the study, and you make any necessary arrangements with your new institution to rely on the FSU IRB for continuing oversight of the study.
- For additional instructions about continuing review and study modification submissions, refer to the to the Researcher's Guide to RAMP IRB, or to the OHSP Researchers Training slides available in RAMP IRB Help Center.
- To close your study that was never submitted within RAMP IRB, complete and submit to OHSP the closure form located here on the OHSP web site.
- If you have additional general questions not listed above, contact OHSP or refer to the contact information in the left panel, and your question will be routed to OHSP staff for follow-up with you directly. For FAQs specific to student-led research, visit this student-led research FAQs page.
- RAMP IRB is accessible within the myFSU portal as an icon under the myFSU Links section, and you must therefore have a valid FSU user credential. The system is designed to be part of the single sign-on process, allowing FSU credentialed users to conveniently access RAMP IRB along with other systems within a single platform using one set of login credentials. Use any of the means below to access the RAMP IRB system to submit your study:
- Sign on to the myFSU portal https://www.my.fsu.edu/portal and click on the RAMP icon;

- Visit https://myramp.research.fsu.edu; or,
- Click on the link from an auto-generated email from ramp-irb@fsu.edu
- If you are experiencing technical issues accessing RAMP, try clearing your browsers' history and/or use another internet browser, then try logging into RAMP again. If the issues continue, contact the FSU RAMP Support Office at RAMP-Grants@fsu.edu or dmullins2@fsu.edu.
- Sign on to the myFSU portal https://www.my.fsu.edu/portal and click on the RAMP icon;
- Non-FSU researchers are generally not provided with access to any RAMP module, including RAMP IRB. However, in very limited circumstances, non-FSU individuals who are collaborating researchers may request Sponsored Guest Access. Such requests must first be formally approved by Department Chair, Associate Dean or designee, and then cleared by OHSP/IRB. All requests for a Sponsored Guest access must be submitted to the RAMP-Grants@fsu.edu mailbox with the subject: Sponsored Guest Access Request (see this RAMP Support Office link for additional information and instructions). The OHSP/IRB will not clear such requests unless the FSU PI has already submitted an IRB application for review, and the application includes sufficient information about the non-FSU researcher(s).
- IMPORTANT NOTE: The RAMP IRB SmartForm application uses business logic to guide you in completion of your submission. The Smartform application also includes on most pages and sections many ? (or Help) icons and buttons to provide you with key instructions and information about what is required to be answered, uploaded or provided for purposes of review, and where to obtain templates and forms. Save time and effort and click on these ? icons to get answers and directions! See sample images below:


- Video tutorials are available to assist you with using RAMP IRB and navigating a study submission workspace. Tutorials last just a few minutes and include video captures of RAMP IRB workspaces. To access the tutorials go here.
- When study documents (e.g., study protocol, consent forms) must be submitted, many templates are available, each tailored to the scope and complexity of your research; these are found under the IRB, Library and Templates tabs. Note that when using protocol templates, some sections may not be applicable to your research; if so you may mark as “NA”, but do not delete the section, otherwise your protocol will be returned to you for correction.
- For step-by-step instructions, refer to the Researcher's Guide to RAMP IRB, or to the OHSP Researchers Training slides available in RAMP IRB Help Center.
- For other questions about using RAMP IRB, including obtaining assistance with using RAMP IRB, visit this general RAMP IRB FAQ web page, also available at: https://ramp.research.fsu.edu/faqs/irb/
- Respond to a Clarification Request in RAMP IRB, not by email or telephone call since these responses cannot be entered by staff into the RAMP regulatory file for your study. In the RAMP IRB submission workspace for the study, under the “History” tab, carefully review the requested clarification(s). When you are ready to provide the requested information and documents, under Next Steps click “Edit Study”, make any requested changes to the SmartForm and/or upload requested documents, and then click “Exit” on the SmartForm once all revisions have been completed. Be certain to click “Submit Response” on the study workspace (to the left of the workflow diagram) and then click OK. Only then will your submission return to our queue for further handling.
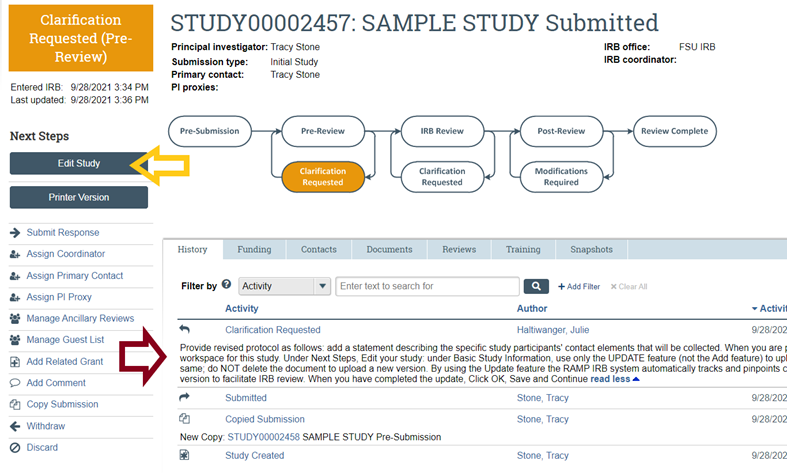
- Do not attach study-related documents to a comment or response submission since these materials cannot be readily accessed or finalized by the IRB or maintained with other study documents when the documents are located outside of the proper submission or SmartForm location or section.
- In limited circumstances and only as an interim measure you may be instructed to provide the IRB with requested information or study-related documents as an attachment to a comment or response submission; note however that you will still be required to provide those documents in the correct workspace or SmartForm location when your study is made available for you to edit so that these materials may be finalized by the IRB.
- To submit a Continuing Review, log into RAMP IRB and under the IRB and Help Center tabs refer to the IRB Researcher's Guide on p. 11 for instructions on creating and submitting a Continuing Review to renew your IRB approval before IRB approval expires.
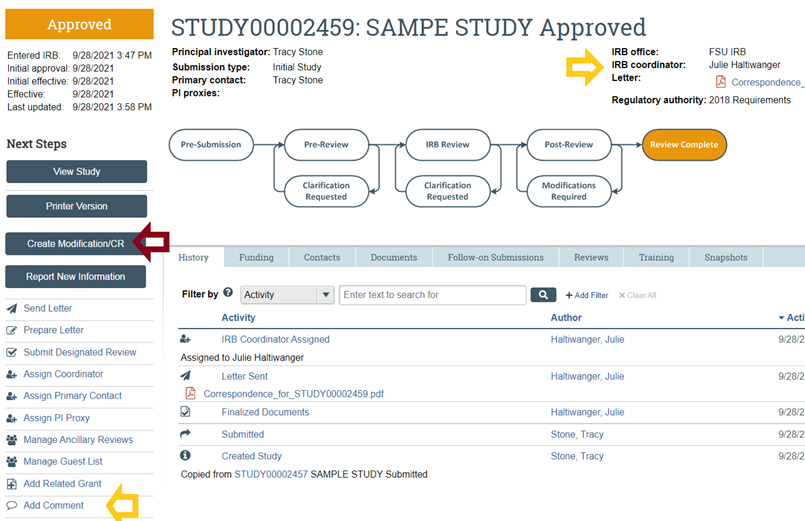
- If you have additional questions use the “Add Comment” feature (to the left of the workflow diagram and listed below under Next Steps) on your study workspace to ask your question and select the option to send an email notification about your question to your IRB Coordinator (whose name is listed above and to the left of the workflow).
- To make a change to or revise your study: log into RAMP IRB and under the IRB and Help Center tabs refer to the to the IRB Researcher's Guide on p. 11, then follow the instructions on submitting a Modification. Note the modification scope “Other parts of the study” will allow you to edit any of the original SmartForm sections except for study team member information; if you need to make changes to both study team member information and another section of the SmartForm, then both scopes should be selected.
- Was your study determined to be exempt from IRB review? If so, then in the official exemption determination letter that you received for this study you will find a list of planned study changes that do not and do require submission for review. Look over these examples to see if your proposed changes will require review. If so or if you are not sure, follow the instructions on p. 11 of the IRB Researcher's Guide to create and submit a modification for this study.
- To close out your study: log into RAMP IRB and from the Help Center refer to the IRB Researcher's Guide on p. 11, then follow the instructions for submitting a Continuing Review (which process is also used to close a study). Note that in order to close a study, the first 4 research milestones in section 4 on the Continuing Review/Study Closure page must be checked. BE sure to acknowledge that the study will be closed, discard any open follow-on submissions related to the study, complete any remaining items or respond to any other questions, attach any supporting documents as may be needed, then select Save, Finish and under Next Steps select Submit. Your Study Closure submission will move into the queue for review and further handling.

- If you have additional questions use the “Add Comment” feature (to the left of the workflow diagram and listed below under Next Steps) on your study workspace to ask your question and select the option to send an email notification about your question to your IRB Coordinator (whose name is listed above and to the left of the workflow).
FSU researchers:
- CITI training completion documentation is synced with RAMP IRB each evening through a nightly data transfer. You should wait for the next day after CITI training course completion to add yourself to a RAMP IRB submission.

- Your CITI profile must:
- Affiliate with FSU, and
- Be accurate, including using your official FSU email address as the primary email.
- The data transfer feature is a courtesy to the FSU research community, and made possible by separate arrangement between the 2 proprietary vendors that support CITI and RAMP IRB. However, if your CITI training completion documentation does not transfer, then you will be required to upload the CITI training completion documentation within the RAMP IRB submission workspace.
- Refer to the Investigator Manual for information about CITI human subjects research training requirements or visit our website’s training page for step-by-step instructions on how to enroll in the correct training: https://www.research.fsu.edu/research-offices/ohsp/investigator-resources/citi-training-requirements/. Only the specified training will suffice for IRB review purposes.
Non-FSU researchers:
- You must provide the FSU Principal Investigator with a copy of your CITI training completion documentation, and the FSU Principal Investigator must upload the copy in other study documents within the RAMP IRB submission workspace.
- Direct questions about funding or grants to your contact in the Sponsored Research Administration (SRA) since those questions are most likely to be associated with the RAMP Grants module, which the OHSP/IRB does not manage; if you are unsure who to contact in SRA, contact their support desk at: RAMP-Grants@fsu.edu. Note however that for ANY IRB submission for which you have documentation of funding, you must add the related grant or funding information in your IRB submission since special IRB-related requirements apply to grant-funded studies.
- Always log in to RAMP IRB and view your study submission’s workspace to check the status of your submission. The workflow diagram and the History tab entries will indicate your study’s submission status. In the figure below, the example workflow diagram indicates that your study is in the Pre-Submission state (the study has not yet been submitted to OHSP and IRB for their review), and under the History tab it shows that you have created (but not submitted) your study.
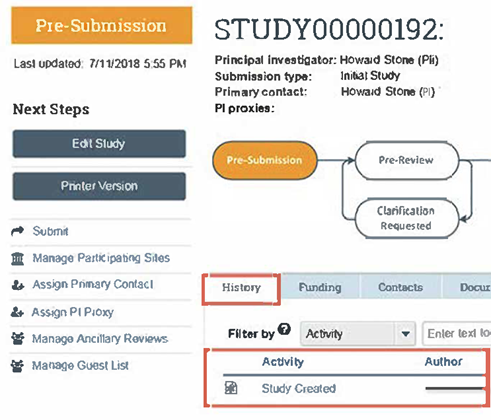
- For a more detailed explanation about using the workflow diagram to check the status of your submission, click on this RAMP IRB workflow explainer.
- Be sure to follow instructions on the Final Page in the RAMP IRB SmartForm for your study, which states that you must “click Submit” in order for your study to ever enter the IRB review queue. With many thousands of FSU researchers, the IRB does not search out for studies in the Pre-Submission stage to follow up for reasons in delay as there may be a myriad of reasons why study staff maintain their studies in the Pre-Submission.
- Note that only these individuals who are authorized to actually submit to the IRB (i.e., Principal Investigators (PIs) or PI proxies) will be able to see the “Submit” button. If you are not the PI or PI Proxy for the study, then you will not be able to see nor execute the “Submit” feature. The study PI or PI Proxy needs to log in; they will see the “Submit” button above the “Assign Primary Contact” button. The PI has the option to “Assign PI Proxy” so therefore they can click Submit or they assign another study team member as PI Proxy; that study team member will then have the authority to click “Submit” on the PI’s behalf for this study.
- Special note if you receive the notification “Response Time Exceeded”: these notifications are only intended as a courtesy to alert you that it has been 14 days since clarification was requested on your submission and our office has not yet received your response. You may still make or submit edits and take the time that you need to do (unless you have been specifically told about a deadline for re-submission; check your correspondence from IRB). You will continue to receive these notifications until you take the required action noted in the Clarification Requested instructions.
- Refer to our Human Research Review web page to learn about IRB turn-around times. The referenced anticipated turn-around times apply only to submissions that are complete (required forms, materials, information and requested clarifications have been submitted or completed, including completion of the required CITI human subjects training for all study staff). Carefully review ALL requirements before submitting otherwise you will risk needless further delay that could have been anticipated and avoided.
- If you have about the status of your submission, contact OHSP use the “Add Comment” feature (to the left of the workflow diagram and listed below under Next Steps) on your study workspace to ask your question and select the option to send an email notification about your question to your IRB Coordinator (whose name is listed above and to the left of the workflow); there, in response to the question about who should receive the email notification about your comment, select "IRB Coordinator." This will help to ensure that the IRB Coordinator assigned to your submission will receive a notification that your comment has been posted in your RAMP IRB workspace. Alternatively and if you have not yet submitted your study (i.e., your study is still in the Pre-Submission status) or an IRB Coordinator has not yet been assigned to your submission (i.e., no name is listed next to "IRB Coordinator"), contact OHSP or refer to the contact information in the left panel, and your question will be routed to OHSP staff for follow-up with you directly.
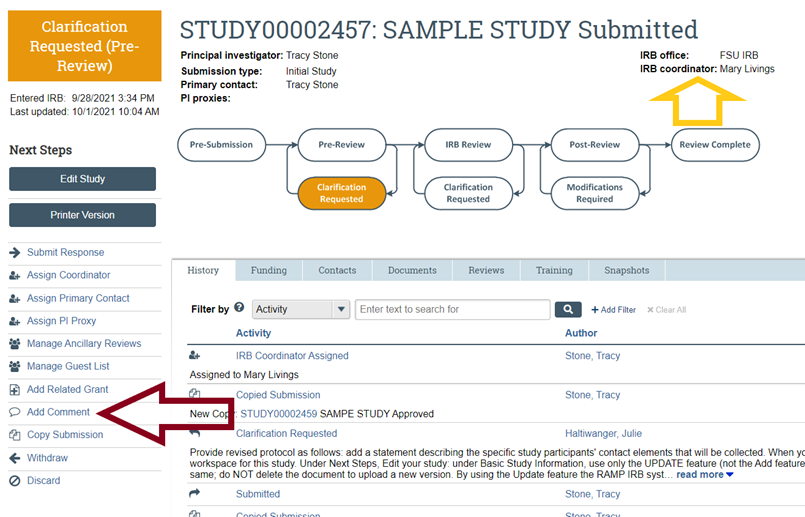
- If you have a RAMP IRB-related question not listed above, contact OHSP using the “Add Comment” feature (to the left of the workflow diagram and listed below under Next Steps) on your study workspace to ask your question and select the option to send an email notification about your question to your IRB Coordinator (whose name is listed above and to the left of the workflow); there, in response to the question about who should receive the email notification about your comment, select "IRB Coordinator." This will help to ensure that the IRB Coordinator assigned to your submission will receive a notification that your comment has been posted in your RAMP IRB workspace.

- Alternatively and if you have not yet submitted your study, contact OHSP or refer to the contact information in the left panel, and your question will be routed to OHSP staff for follow-up with you directly.
- For RAMP IRB-related FAQs specific to student-led research, visit this student-led research FAQs page.
- Generally, YES (a few exceptions may apply; see exceptions below).
- *Preliminary research activities may include procedures, processes, testing, trials and other actions undertaken with the intent to assess and refine or modify a research plan or other aspects of the research (e.g., design; approach; methods or activities; instruments or measures; test products; researchers and study teams’ roles and responsibilities; recruitment; consent; interventions or interactions with human subjects; and data extraction, collection, analyses or storage), and which activities are undertaken prior to performance of the main or larger study. Sometimes these activities may variously or ambiguously be referred to as piloting, feasibility or practice activities.
- Preliminary research activities are subject to required IRB or other human research regulatory review when those activities meet the definitions of research and human subjects as those terms are defined under federal regulations.
- Research means a “systematic investigation, including research development, testing, and evaluation, designed to develop or contribute to generalizable knowledge”, and generally refers to a planned study employing empirical principles and methodology to formulate, examine or test hypotheses, collect data or information, characterize phenomena, acquire knowledge and interpret results. Generalizable knowledge refers to results or findings that have relevance beyond the individuals, sample or program from which data or information are collected, including information that is added to the literature.
- Human Subject means a living individual about whom a researcher (whether faculty, staff and students, as well as contractors) obtains information or biospecimens through intervention or interaction with the individual OR obtains (even in the absence of intervention or interaction) identifiable private information or identifiable biospecimens related to an individual, for a research purpose.
- Note that the applicable regulations make no reference to an activity’s size or scale in the definition. Non-research projects can be large in size and scale; research projects may be small in size and scale. Additionally, the intent to publish, present or otherwise disseminate or share results is not a determining factor for whether an activity involves research; the findings from many activities that may not meet the definition of research may still result in the finding’s publication or dissemination. Note also that the regulations make no reference to whether information or biospecimens collected from living individuals are added to the main or larger study; the act of simply obtaining an individual’s information or biospecimen as part of preliminary research is categorically done for a research purpose.
- Due to their nature and intent to test, assess and refine or modify a research plan or other aspects of the research, preliminary research activities will require IRB or other human research regulatory review WHEN those activities involve researchers’ interventions or interactions with living individuals about whom information or biospecimens are collected, or involves (without intervention or interaction) researchers’ obtaining individuals’ identifiable private information or identifiable biospecimens.
- Preliminary research activities should always be described in the research protocol as well as carefully and prominently distinguished from the main or larger study activities. The description should include an explanation about how preliminary research activities will be used to test, assess and refine or modify the research plan or other aspects of the research, as well as how if at all human subjects will be involved and their data used. If human subjects will be involved, the recruitment and consent process (including the consent form) must inform human subjects that they will be involved in preliminary research activities and how their information or biospecimens will be used, and whether their data will be added to the main or larger study and retained, destroyed or deidentified.
- Exceptions: under limited circumstances, preliminary research activities may not require IRB or other human research regulatory review because they are not part of a systematic investigation or because the activities do not involve human subjects. These may include, for example:
- Visiting or evaluating a potential site to see if the research is possible.
- Going through a consent process with friends to see if the information is comprehensible.
- Sharing your survey or interview instrument with a few experts in the field for their feedback as to whether the questions are appropriate for the topic and/or study population.
- Asking an expert for their advice or guidance regarding how to monitor an intervention or procedure for a study population’s safety.
- Soliciting feedback or an opinion from a colleague or mentor about research design.
- Consulting a community advisory board or an organization’s leadership about what you propose to study or how best to conduct your study involving their constituents.
The above exceptions may be subject to change, and other examples may be added to the list above.
- If there is any question about whether preliminary research activities will require IRB or other human research regulatory review, submit in RAMP IRB a completed HRP-503d - Template Determination Form and related materials; OHSP will review the form and related materials and let you know whether further IRB review is required and if so, what additional information and materials must be submitted. The HRP-503d Template Determination form is accessible by logging into RAMP IRB, and navigating to the IRB, Library and Templates tabs. See this Request a Determination video tutorial to see how to submit the HRP-503d form in RAMP IRB.
Usually yes. Review of individuals' previously collected information or biospecimens may require IRB or other human research regulatory review. To be considered previously collected information or biospecimens, the information or biospecimens must be "on the shelf" at the time the research is proposed. Some existing data studies that utilize a source that is publicly available or that do not have any identifying information may be excluded from the definition of research involving human subjects, and may therefore not require IRB review. In order to determine whether your proposed research may require IRB review, please contact our office for assistance.
Students may be involved as study subjects, but particular care must be taken when students are not of majority age (in which case their parent's permission is required) and because there is an inherent power differential between the students and the faculty, instructors or supervising staff who may serve as Principal Investigators and/or members of research teams. This differential may create undue influence or be perceived to coerce students to participate in research projects. No matter how well-intentioned, students may feel compelled to participate believing that failure to do so will negatively affect their grades and the attitude of the instructor or staff (and perhaps other students) toward them. For this reason, the IRB may not, in general, permit an instructor to use his/her own students as subjects in the instructor's research project. The IRB will review whether collection of data by a third party or a student's consent (and parental permission) to use his/her own data be obtained after grades are entered, as possible ways to mitigate concerns regarding this type of research.
The IRB will also carefully consider whether students are being recruited or involved because they are a convenient and accessible sample as opposed to representing a chosen target group for research inquiry.
Yes. Research conducted in schools may require District School approval, Principal of school approval, or District IRB or review board approval, depending on whether the school is public or private and on the requirements of a particular school district. Leon County Schools requires approval at the District level (see Hints for Researchers), and has a review board that determines whether the study can be conducted using Leon County students as research subjects (including data collection or analysis). Depending on the nature or design of the research, teacher consent may also be required. Note that parental permission and child assent must always be obtained, unless an appropriate waiver has been granted by the FSU IRB. All such school approvals shall be submitted to the IRB for inclusion in the protocol file. The IRB may require district approval as a condition of FSU approval, or may permit the researcher to obtain district approval at a later time, as long as proof of school approval is submitted as required by the IRB.
The FSU Laboratory School also requires that researchers obtain FSU Laboratory School permission/approval prior to engaging in any research activities involving its students. Please contact the FSU Laboratory School directly to request this permission.
Research involving the derivation and use of human embryonic germ cells from fetal tissue may be conducted with Federal support. Research on existing human embryonic stem cell lines may be conducted with federal support if the cell lines meet the US President's criteria which he announced on August 9, 2001 (http://grants.nih.gov/grants/guide/notice-files/NOT-OD-02-005.html). Research involving the derivation of new stem cells from human embryos or the use of human embryonic stem cells that are not listed on the NIH Human Embryonic Stem Cell Registry may not be conducted with federal support.
In Vitro research and research in animals using already derived and established human cell lines, from which the identity of the donor(s) cannot readily be ascertained by the investigator, is not considered human subject research and is not governed by the HHS or FDA human subjects protection regulations appearing at 45 CFR Part 46 and 21 CFR Parts 50 and 56. IRB review is not required for such research.
HHS-conducted or supported research that uses human cell lines where the donor(s) may be identified including cells that retain links (such as a code) to identifying information is generally considered human subject research that is governed by 45 CFR 46 because the donors are human subjects. IRB review and approval is required for such research. Please refer to Stem Cell Guidance for more information.
The following techniques for assuring confidentiality are listed on a continuum according to the degree of prospective harm that may occur:
- Substitute codes for personal identifiers and store the key in a different physical location;
- Remove the face sheet, which typically contains personal identifying information such as name, telephone, address;
- Data with personal identifiers should be kept in locked files, access to the data should be controlled by the researchers with specified procedures;
- Research assistants should be educated in the importance of confidentiality and the potential risks of harm to subjects. In situations of serious risk, assistants could be asked to sign confidentiality agreements;
- Access to data can be controlled electronically, perhaps by storing very sensitive data on computers not attached to a network where hackers could penetrate the files. Electronic files can be protected with key words, and portable computers should be appropriately secured;
- The data can be manipulated electronically, for example by encrypting data files;
- The data can also be recoded to eliminate identifiers by collapsing it into categories;
- Research involving many data files on the same person can use anonymous linkage systems; and/or
- Researchers may apply for a federal National Institutes of Health (NIH) Certificate of Confidentiality (CoC) or other federal agency equivalent to protect sensitive information from subpoena or other legal processes or governmental agencies. The U.S. National Institute of Justice (DOJ) provides guidance about when a Privacy Certificate is required. To find out more about obtaining a CoC from the NIH or other federal agencies, see our Confidentiality panel under Special Topics on this Consent Process page).
For additional guidance on de-identification for protecting subjects' confidentiality, refer to this U.S. Department of Health and Human Services guidance, applicable to protected health information under the HIPAA Privacy Rule but perhaps relevant to subjects' confidential information more generally.
Special Note for National Institutes of Health (NIH)-funded Research or Applications for NIH Funding. The NIH has issued a new NIH Data Management & Sharing Policy which will go into effect for proposals for NIH funding that are submitted for due dates on or after January 25, 2023. Under the policy, researchers must have in place or submit a data management and sharing plan, to include provisions to secure and protect human subjects' data, including a description about how shared human subjects' data may be protected, such as in the informed consent process and materials; de-identification procedures; and use of the NIH Certificate of Confidentiality (CoC). For further information, refer to our NIH Data Management and Sharing (DMS) page [link].
- Many incidents (e.g., adverse events, breach of confidentiality, awareness of abuse/neglect/misconduct, protocol deviations) require reporting to the IRB within business 5 days from when researchers become aware of the incident. Refer to page 3 on our HRP-214 - Reportable New Information form to see what incidents require reporting; refer also to the section, "What are my obligations after IRB approval?" in the Investigator Manual.
- To submit a Report of New Information: log into RAMP IRB and under the IRB and Help Center tabs refer to the IRB Researcher's Guide on p. 13, then follow the instructions on creating and submitting a Reportable New Information.
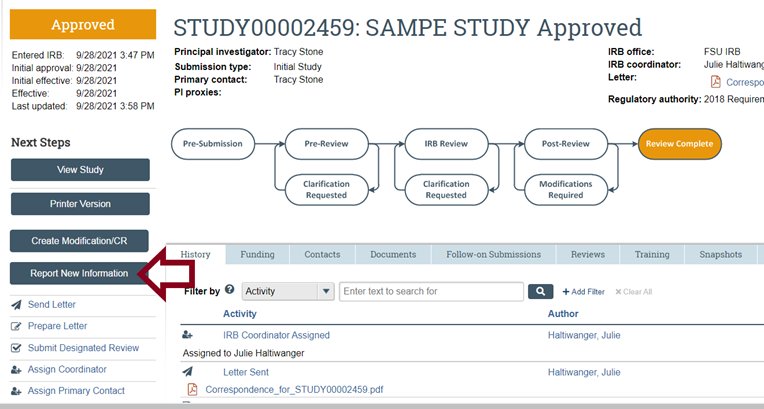
- Only if RAMP IRB is not accessible because of scheduled downtime or other circumstances and due to which you may miss the required reporting to the IRB within 5 business days, you may complete and send by email (humansubjects@fsu.edu) attachment a Report of New Information (RNI) (HRP-214 - FORM - Reportable New Information). The RNI will be acknowledged and reviewed and, as needed, you will be contacted for further information and instructions (which may include resubmitting the RNI through RAMP IRB once accessible).
- The OHSP office does not recruit research participants; rather, the office is responsible for the regulatory review and monitoring of FSU research studies involving human subjects. However, depending on the type of research in which you are interested in participating, you may consider looking over one of the FSU research centers and/or institutes (list located at this link) and then using their contact information to inquire about available studies. Also consider looking over our own guidance for prospective research participants: Becoming a Research Participant | FSU Office of Research
Yes, a CAMS COI submission will affect review of an IRB submission in one or more of the following ways:
- Any RAMP IRB submission involving FSU employees or agents may result in a separate CAMS system notification to the employee or agent of their need to satisfy a CAMS disclosure and/or reporting requirement. If so, the employee or agent should take prompt action in CAMS to satisfy these requirements in order for a RAMP IRB submission to proceed to human research regulatory review.
- Note that each RAMP IRB submission for a new study, or a study modification involving addition of study staff for an active study, may require a separate CAMS disclosure and/or reporting for each study staff. This is not intended to be duplicative: applicable law requires that each FSU employee or agent's disclosure or reporting of financial or other interests be separately evaluated for its relation to and affect upon the design, conduct and reporting for each study (not all studies collectively) in which they may be involved, as an interest's relation or affect may differ among studies (e.g., a researcher's disclosure or report of a financial interest related to or possibly affecting one study may be unrelated to or have no affect upon another study in which the researcher may be involved; such distinct findings require separate documentation).
- An IRB submission WILL NOT proceed to IRB review if the COI tab or COI “Status” that appears in your study workspace for any member of the study team is any of the following: (a) Team Member(s) without Certifications; (b) Awaiting Profile Update; and (c) Administrative Review (see image below).
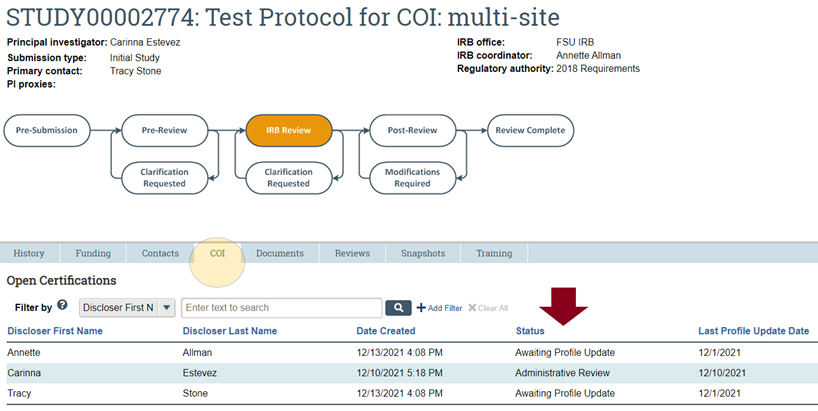
ANY of the above statuses pertaining to any member of a study team above will delay IRB review of a submission in the RAMP IRB queue. You are strongly advised to promptly tend to any CAMS submission for which one of the above statuses exists in order to avoid unnecessarily prolonging IRB review of your RAMP IRB submission.
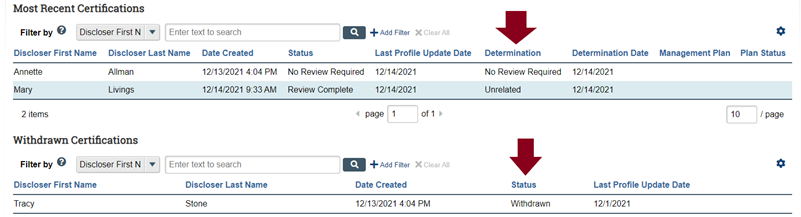
- An IRB submission may proceed to IRB review if the COI “Determination” or “Status” that appears in your study workspace for every member of the study team is one or more of the following: (a) No Review Required; (b) Unrelated; or (c) Withdrawn (see image below).
- If a determination is made that a member of a study team has a conflict of interest, direct and specific disclosure to research participants (human subjects) about such interests is required. Such disclosure must be included as part of the informed consent process, and may be conveyed as follows: in the first paragraph in the “What else do I need to know” section of the HRP-502 consent form, after the first paragraph in the “What is this study about” section of the HRP-502a or HRP-502c consent forms, or at the end of the first paragraph in the HRP-502i Information Sheet form. The IRB may require additional language. Refer to our HRP-502COI – TEMPLATE COI Consent Language Template in RAMP IRB (or here on the OHSP Templates & Required Forms web page; scroll down to the Consent-related Templates section and look for the Conflicts of Interest Consent Language Template) for different types of interests to disclose to human subjects. Any of these forms missing such language will be returned to the study team for correction.
- The IRB will not approve of an IRB submission (new, continuing review or modification) unless any related COI management plan is deemed by the IRB as having satisfied federal regulatory criteria for approval of research. This includes revisions to a COI management plan, as well as implementation of informed consent procedures through which study subjects are adequately informed about investigators’ conflicts of interest (see bullet above). Note that the IRB may regardless of any other FSU office’s conflict of interest review outcome determine that a conflict of interest does exist; require additional information about researchers’ interests; impose additional requirements in order to minimize such conflicts; require that study subjects be informed about such interests or conflicts; and take any other actions to ensure that human subjects are both protected from research risks implicated by a conflict of interest as well as informed about such conflicts of interest.*
- Submission in CAMS of any new or change in a COI disclosure for any study team member for an existing IRB study may, if a COI management plan is imposed based upon the submission, require a RAMP IRB study modification. If a study modification is not initiated by the PI and the OHSP/IRB becomes aware of the new or changed COI disclosure, the PI will be contacted to submit a study modification.
- COI disclosures reported in a RAMP IRB submission before implementation of the CAMS COI module will appear in the Basic Study Information section (Question 7) and/or Local Study Team Members section (Questions 1 and/or 2 for external team members) of your RAMP IRB SmartForm or workspace as depicted in the images below:
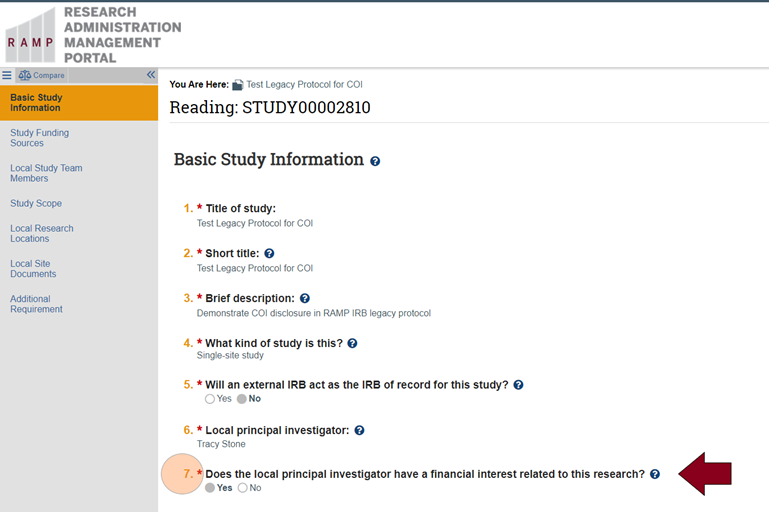
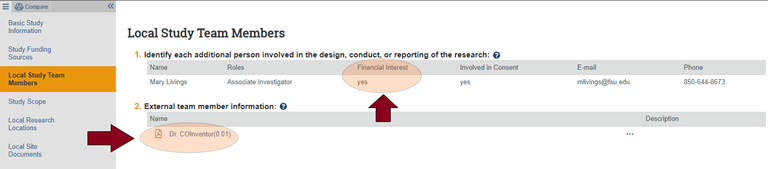
Note that the “?” button for Question 7 above provides related information and instructions as depicted below:
Review of these pre-CAMS RAMP IRB COI disclosures may be handled similarly as those that are reported in CAMS, including the requirement that human subjects be informed about the conflict of interest. Refer to our HRP-502COI – TEMPLATE COI Consent Language Template in RAMP IRB (or here on the OHSP Templates & Required Forms web page; scroll down to the Consent-related Templates section and look for the Conflicts of Interest Consent Language Template) for different types of interests to disclose to human subjects.
- Check out our Conflicts of Interest/CAMS web page for an overview, definitions, references and other helpful information.
- Authority for and support of CAMS and FSU conflicts of interest reporting requirements are provided by the FSU Office of Compliance and Ethics and the FSU Office of Research Compliance Programs, respectively, NOT the OHSP or IRB! For your questions or to obtain information about the CAMS COI system, including an overview, announcements, training, support and contacts, visit this CAMS project page.
- CAMS is accessible within the myFSU portal as an icon under the Links section, and you must therefore have a valid FSU user credential. The system is designed to be part of the single sign-on process, allowing FSU credentialed users to conveniently access CAMS along with other systems within a single platform using one set of login credentials. Use any of the means below to access the CAMS system to submit your study:
-
- Click on the CAMS icon within the myFSU portal; or
- Click on the CAMS link within an Outlook email notification (which occurs when you need to take action in the system).
- Type the CAMS website address in your internet browser: https://cams.fsu.edu
- To find out how to navigate through CAMS, click the "CAMS Quick Start Guide" or access the guide at this CAMS Project page, see the video "How to Update Your Disclosure Profile" or access the video in the COI/CAMS module Help Center, or submit a support ticket to the CAMS Support team.
IMPORTANT NOTICE:
Federal law provides the IRB with authority to, for example, review human research; require modifications to secure approval; and ensure that legally effective informed consent will be and is obtained, including disclosure of the above interests. FSU institutional officials may not approve of research involving human subjects that has not been approved by the IRB, including IRB approval of or required modifications for COI consent language (see 45 CFR §§46.109, 46.111(a)(4), 46.116; 42 CFR §50.605(a)(1)(ii); and 45 CFR §94.5(a)(1)(ii)).
Yes. Additional federal regulatory requirements may apply when human research involves a "clinical trial." Refer to the U.S. Department of Health and Human Services [link] and the U.S. Food and Drug Administration [link]) definitions. Refer to our draft Clinical Trials/Human Research Workflows algorithm for an illustration of how clinical trials may be reviewed at FSU.
With perhaps a few slight distinctions, a clinical trial generally refers to a research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of the interventions on biomedical or behavioral health-related outcomes. Below are some of these additional requirements.
Consent forms. Consent forms used in clinical trials must include the following:
- Identify any expenses that study participants will incur. Describe whether participants or their insurance will be billed for clinical trial-related services, items or other expenses, as participants may incur deductibles, co-pays, co-insurance or full pay.
- Research-related injuries. Describe how participants will be referred for necessary medical care in the event of any clinical trial research-related injury, and describe whether and how such participants will be compensated for those injuries.
- ClinicalTrials.gov registration. Provide if required by the study's federal sponsor or FDA a statement to the effect that the clinical trial will be registered in the federal government's ClinicalTrials.gov web site. Use the FSU approved consent form's template language. Consent templates are available in RAMP IRB, under the IRB, Library and Templates tabs.
- Publicly post the consent form. For any study that is supported by federal department or agency funding and for any applicable clinical trial under FDA regulations, an unsigned copy of the study consent form approved by the IRB must be posted to a publicly available federal website after the research has been closed to recruitment and no later than 60 days after the last study visit of any subject.
Provisions to Monitor Study Data to Ensure Study Participant's Safety. The study protocol must include these provisions. Refer to section 18 of the FSU approved template protocols. Protocol templates are available in RAMP IRB, under the IRB, Library and Templates tabs. Refer also to the NIH policy and related guidance on data and safety monitoring.
FDA requirements
The Food and Drug Administration (FDA) regulations apply to many clinical trials involving drugs, devices, or biologics and nutritional supplements, and may require submission of special IND applications (for drugs, biologics) or IDE applications (for devices) before submitting the study for FSU IRB review and beginning the study. The IRB will require this documentation in study protocols submitted for IRB review and in related attachments. See section 5 of the FSU approved template protocol, HRP-503 - TEMPLATE PROTOCOL.
Training
The study team will be subject to FSU training requirements. This includes completion of initial and refresher CITI training in human subjects research, and may also include completion of Good Clinical Practice (GCP) training. Visit our CITI Training web page to learn more.
Clinical Trial Assistance. FSU's Office for Clinical Research Advancement (OCRA) provides other guidance, tools, resources and facilitation to assist the human research community with meeting applicable clinical trial-related requirements; click this OCRA webpage for details and contact information.
Need More Information. Be sure to visit our Clinical Trials page, check out our draft Clinical Trials/Human Research Workflows algorithm, and visit the NIH Clinical Trials webpage to learn more!
Post approval compliance monitoring (PACM) is a procedure by which the FSU Institutional Review Board (IRB) conducts an assessment of an IRB-approved or cleared study to ensure that a researcher is adhering to legal, ethical and policy requirements for protection of the rights and welfare of study participants as well as the conditions of IRB approval or clearance for the study. PACM may help to prevent incidents of serious non-compliance, or correct those that have already taken place, especially before regulatory agencies and study sponsors find non-compliance and possibly suspend studies or withhold funding.
All studies approved by the FSU or an external IRB or otherwise cleared through human research regulatory review are subject to post approval compliance monitoring, and all Principal Investigators (PIs) and their study teams must fully cooperate with monitoring as a condition of their study's approval or clearance. Most PACM involve routine assessments, in which studies are randomly selected for review, largely based upon a study's regulatory classification, risk or study population. Some assessments involve directed or for-cause reviews; these may result due to concerns about the rights and welfare of study participants or requests by institutional leadership, study sponsors or federal and state agencies. If your study is selected for PACM assessment, you will be informed about the reason for the assessment.
For more detailed information about post approval compliance monitoring, including the FSU standard operating procedure for PACM and the checklists that may be used to assess studies, visit the OSHP Post Approval Compliance Monitoring program web page [link].
YES and NO.
The requirements may differ because research conducted in countries other than in the U.S.* are subject to those countries’ legal requirements as well as cultural norms and customs with which researchers, including researchers from FSU, may be expected to conform. These requirements may differ in significant ways from those that apply to studies conducted in the U.S. See this federal agency compilation of international standards for human subjects protection, covering all regions of the world and most countries.
And regardless of whether research is conducted abroad or in the U.S., U.S. federal laws that govern human research will apply to the research. The application of these federal laws may depend upon the research institution under whose auspices the research is conducted, as well as the federal department or agency that supports or funds the research. Some examples of U.S. requirements that apply to U.S. human research and that may apply to international research include the following:
- Federal Policy for the Protection of Human Subjects (aka the “Common Rule”) [link]
- Food and Drug Administration regulations [link]
- Federal acquisition (contract) regulations [link]
- Laws and policies of other federal departments or agencies that sponsor or fund the international human research [link; scroll down to Common Rule Departments and Agencies]
Thus, international research may be subject to overlapping, competing or complementary requirements. For FSU researchers (faculty, staff or students) that will conduct international research, the FSU IRB will apply the same ethical, regulatory and FSU policy requirements to international research that are applied to research that may be conducted in the U.S., in addition to any requirements of the host country where the research will be conducted. By law, the most stringent of these requirements must be applied.
*Note that research that includes virtual interactions or interventions between researchers located in the U.S. and human participants who are located in countries other than the U.S. is considered international research.
Below are key considerations that the FSU IRB will take into account when applying both U.S. and non-U.S. requirements in the IRB's review of international research involving human participants. Additional considerations may be added depending on new regulatory developments, so check back frequently to stay current. Click on the linked consideration to learn more. Click on the ^ button to return to the top of this list.
- Embargoed Countries and Other International Restrictions
- Obtain FSU IRB Approval in Advance
- Qualifications of the Study Team
- Local Review and Approval
- Consent Process
- Translations and Local Interpreters
- Compensation or Participation Incentives
- Children as Study Participants
- Information and Data Security
Embargoed Countries, Foreign Influence and International Travel Restrictions
Conducting international research involving countries for which a U.S. embargo or sanction is in force may be subject to certain restrictions, including for example exports, imports and other transactions in goods and services; foreign influence; sharing of research information; and travel restrictions. While the FSU IRB does not in its review of international research enforce any of the University’s requirements or restrictions related to embargoed or sanctioned countries, foreign influence, international travel or related restrictions, researchers are responsible for consulting with the FSU Office of Research Compliance to see that any international research activities, including travel, are permissible. [^]
Obtain FSU IRB Approval Well in Advance
Whether conducted in the U.S. or abroad, human research conducted by FSU researchers (faculty, staff or students) may not begin without advance FSU IRB approval; researchers must obtain IRB approval before their study can begin. Retroactive (after a study has begun) IRB reviews or approvals are not permitted by law; studies that are started or undertaken without advance IRB review and approval are considered non-compliance with federal law and researchers will be sanctioned. To account for the usual IRB human research review process and turn-around time as well as the additional time and effort that may result from special requirements (refer to some of these below) imposed upon international research, take steps to obtain IRB approval before you leave the U.S.
Importantly, in your application for IRB review and your protocol (using Section 24 (“Setting”) of the FSU approved protocol template (link to RAMP IRB protocol templates)), provide a complete, detailed description of where the research will be conducted and provide, if not included elsewhere in the protocol, information about how the following key considerations will be handled. [^]
Qualifications of Researchers and Study Team Members
Whether a study is conducted in the U.S. or in foreign countries, a criterion for IRB approval of a study is that researchers and other study team members affiliated with the study are suitably qualified to conduct the study, including having the knowledge, skills and abilities to ensure that study participants will be adequately protected against research risks.
- Researchers are expected to document, in any study submitted for the IRB’s review, that all study staff have the requisite qualifications to conduct the study, including: training in the methods being used in the study and the population and/or condition under study; human subjects protection training; and for clinical trials or studies involving FDA-regulated products, good clinical practice or similar training.
- Additionally for international research, researchers must demonstrate that they are appropriately qualified to carry out study activities in the country or region of research interest. For this purpose, Principal Investigators, including faculty advisors of student researchers, are responsible for conferring with FSU office and institutional officials about plans to conduct international research; consulting with local community and/or institutional leaders and officials who are knowledgeable and experienced about the local laws, regulations and practices where the research will take place; and ensuring that all FSU study team members (particularly student researchers) are adequately supervised when abroad and interacting with study participants.
- The above should be described in Section 25 (“Resources Available”) of the FSU approved protocol template (link to RAMP IRB protocol templates)). [^]
Local (non-U.S.) Review and Approval
Researchers conducting international research involving human participants must always secure applicable local (i.e., non-U.S.) ethics or other review and approval for their study and maintain such documentation.
- Well before requesting FSU IRB review, researchers should carefully investigate the need for local review and approval, including coordinating such requirements with researchers’ local contacts and local collaborating or cognizant agencies or authorities; securing and documenting support of the study, including with letters of permission, cooperation and/or support; and maintaining documentation of local review, approval and support.
- If no local review is required, a statement to that effect should be included in the local letters that are provided by local contacts and local collaborating or cognizant agencies or authorities.
- The FSU IRB will require that researchers provide the FSU IRB with documentation of local ethics or other approval (or no need for approval) and may require documentation of other local support of the study; “pending” local review or approval will not suffice. This requirement is in accordance with federal laws about the applicability of foreign laws, regulations and procedures (see e.g., Common Rule [link], sections 101(g) and (h)). [^]
Whether human research is conducted in the U.S. or in foreign countries, researchers must obtain the legally effective informed consent of the study’s human participants before activities involving the participants may begin. Researchers must also document (e.g., by maintaining copies of study participants’ signed consent material) that consent was actually obtained from each participant.
- Foreign laws, regulations and procedures must be considered for purposes of determining what may be deemed “legally effective informed consent” and researchers should carefully investigate as well as confer with their local contacts and local collaborating or cognizant agencies or authorities to ascertain what consent-related laws, regulations and procedures will apply and how these must be followed. For example, special notice and consent is required for research involving persons that are located in the European Economic Area; refer to our GDPR in Research web page for more detailed information and instructions, specifically the panel "Notice and Consent Requirements").
- The FSU IRB will require that researchers provide the FSU IRB with documentation of the applicable local consent-related laws, regulations and procedures. The IRB will expect researchers to conform to any applicable local requirements and those under the Common Rule, including the most stringent of these requirements. This is in accordance with federal laws about obtaining and documenting informed consent (Common Rule [link], sections 116 and 117).
- When preparing recruitment and consent-related procedures and materials (as well as data collection instruments and other study activities), researchers must carefully follow the applicable local laws, regulations and procedures that relate to recruiting and consenting prospective study participants and involving participants in any study activity. Recruitment, consent, data collection and study activities should use language that is both understandable and familiar to the prospective populations from which human participants will be drawn. Ideally, study materials used with participants should be prepared by researchers who are fluent in the local language; alternatively, these materials may be prepared or authenticated by collaborators who are fluent in the local language. The IRB may consider alternatives when appropriate and if researchers provide, in their applications submitted for IRB review, explanations and documentation about cultural norms or conditions for which alternative recruitment, consent, data collection and study activities may be suitable.
- Consent forms should include local contact information when it is more appropriate for the local study participants (due to access, costs etc.); this may include local telephone numbers and email addresses for local study collaborators, community leaders who are knowledgeable about the study, and/or local ethics or other offices that performed local review of the study.
- The requirement to document that informed consent has been obtained (i.e., having study participants sign a consent form) may be waived by the IRB but only if researchers provide the IRB with documentation sufficient for the IRB to find that the federal regulatory requirements for a waiver are satisfied. Among these may be that researchers will, due to cultural, religious or literacy factors, put into place an appropriate alternate means of documenting informed consent (e.g., short form for consent; verbal consent; witnesses; permission by cultural group or community leaders).
- Detailed descriptions about how the study team will conform to the above requirements must be included in Sections 22 ("Consent Process") and 23 ("Process to Document Consent in Writing") of the FSU approved protocol template (link to RAMP IRB protocol templates).
- For FSU-approved consent-related templates, use this link to RAMP IRB to review and select a consent template; an array of templates are available, based upon different research contexts (e.g., biomedical or social-behavioral studies) and populations (e.g., children or adults). A description of available consent-related templates may be found on our Investigator Resources/Templates & Required Forms page (scroll down to Consent-related Templates). [^]
Translations and Use of Local Interpreters
Effective communication with non-English speaking study participants is essential, and researchers should plan for how communications between participants and the study team will be managed. First, study team members or local collaborators who are fluent in the local language should be reasonably accessible to all study participants. Second, English language and translated foreign language versions of study materials that will be seen, read or completed by study participants must be submitted to the FSU IRB for review.
Additionally:
- Have a local contact verify the translations as suitable for study participants.
- Consider using local translators/interpreters who are knowledgeable about the local community and fluent in the local language/dialect.
- Using interpreters to communicate with study participants is subject to additional considerations, including:
- Translators/interpreters should be trained in and knowledgeable about the protocol and study activities.
- Study participants’ privacy and the confidentiality of their data and biospecimens must be maintained.
- Possible conflicts of interest, including conflicts in relationship between an interpreter and prospective study participants that may lead to coercion, undue influence or bias, must be disclosed; conflicts must be eliminated, minimized or adequately managed.
- Interpreters should be available throughout the study to answer questions, address complaints, or relay instructions throughout the conduct of the study.
- Study-related terms and concepts for which direct translations are not available must be carefully rendered in language appropriate for study participants. [^]
Compensation or Participation Incentives
Compensating or providing incentives for study participants for their time and effort is generally acceptable but must not serve to unduly influence participants. In international research, researchers must determine whether local laws, regulations or policies permit compensation or incentives. Researchers should also carefully consider the local context when establishing the amount of compensation or incentives and the method, timing and frequency in how these may be provided. Researchers should describe for the IRB the relative value of the compensation or incentives (which may include food, goods, supplies and other tangible gifts or items in addition to money) in the local and U.S. currency and to the comparable earnings that study participants may be expected to earn for a similar commitment in time and effort.
The above should be described in Section 13 (“Recruitment Methods”) of the FSU approved protocol template (link to RAMP IRB protocol templates)). [^]
Children as Study Participants
Children are as study participants accorded under federal law with protections in addition to those that apply to adults, including limits on the types of studies in which children may be included, the research risks to which children may be exposed, restrictions on involving children who may be wards of the state, and specific requirements for obtaining parental permission and the assent of a child. For international research involving children, researchers must investigate and obtain authoritative information about what local requirements may apply to research involving children. This information must be provided to the IRB for its review of studies involving international research; specifically, researchers must provide the IRB with local information about the following:
- The age at and the conditions under which parental (or legal guardian) permission (consent) is not required for activities included in the study.
- Requirements for obtaining and documenting parental (or legal guardian) permission and child assent.
- The above should be described in Sections 10 ("Subject Population") and 11 (“Vulnerable Populations”) of the FSU approved protocol template (link to RAMP IRB protocol templates).
Some study activities may result in researchers becoming aware of abuse or neglect of children. Therefore, researchers should be knowledgeable about what local laws, regulations and procedures (e.g., mandatory reporting) may apply under these circumstances, and to plan accordingly, including knowing when and how to report abuse or neglect, and to provide in consent-related materials a statement to the effect that abuse or neglect of children about which researchers may become aware may be reportable to authorities.
Note again that the IRB will require compliance with the most stringent among U.S. federal and local laws, regulations and practices.
The term “children” is defined as persons who have not attained the legal age for consent to treatments or procedures involved in the research, under the applicable law of the jurisdiction in which the research will be conducted. [^]
While it may not always be feasible when abroad or in the field to securely collect and save research data (including data related to a collected biospecimen) to a secure FSU server, every effort should be made to do so. In the protocol included in your application submitted for IRB review, be sure to describe the steps that the study team will take to securely collect, store, transmit and/or share electronic or printed research data, including access control and authorization of access, password protection, encryption and other controls. If using a secure FSU server to save research data is not feasible, provide a rationale. Once you have returned to the U.S., research data should be securely saved and stored, following all applicable FSU as well as School and Department policies (e.g., refer to 4-OP-H-5 Information Security Policy [search for the policy at this link] and 4-OP-F-7 Policy on Safeguarding of Confidential Financial and Personal Information [search at this link]).
- The above steps should be described in Sections 7 ("Data and Specimen Banking") and 17 ("Data Management and Confidentiality") of the FSU approved protocol template (link to RAMP IRB protocol templates).
Note that some countries may restrict or otherwise limit the use or disclosure of identifiable information. As an example, for international research conducted in a European Union (EU) country, research use or disclosure of personal data may be subject to EU law, such as the General Data Protection Regulation (GDPR; see our GDPR in Research web page for more detailed information and instructions). Note as well that use or disclosure of identifiable or personal data may be restricted or prohibited when certain countries are involved; see the above section on Embargoed Countries and Other International Restrictions. Researchers should always consult with their local contacts about any applicable local laws, regulations or procedures that may limit the use or disclosure of a study participant’s information. [^]
Almost all sponsors of grants, contracts and cooperative agreements require that funding applicants or recipients demonstrate compliance with federal laws for protecting human subjects. Funding applicants must provide a thorough description about how human subjects (study participants) will be protected against study risks. Funding recipients must certify, before funds may be used, that an IRB has approved of their human subjects research activities.
Refer to our Grants Applications, Funding & the IRB to access much more detailed information, guidance, instructions and links to resources related to sponsors' pre- and post-award human subjects and IRB-related requirements.
Maybe, but note that when permitted by applicable federal regulation*, FSU and TMH will carefully work together and seek to avoid duplicate IRB review whenever possible. The FSU and TMH IRBs have mutually concurred to following certain processes for determining when either FSU or TMH will perform IRB review, and which IRB will do so.
To make this regulatory determination, both the FSU and TMH IRB offices must first conduct an administrative (or "pre-IRB") review. Study teams will be need to submit certain study materials in the separate FSU and TMH research administration systems (at FSU, called RAMP IRB) for purposes of this administrative review.
Most important, FSU researchers must first carefully review the TMH IRB web page to see what TMH requirements will apply, then contact the TMH IRB office to see how to satisfy those requirements. Afterwards, FSU researchers should prepare their studies for FSU administrative review.
FSU researchers are strongly advised to also contact FSU's Office of Clinical Research Advancement (OCRA), which is charged with assisting researchers whose clinical collaborations involve non-FSU partners such as TMH; click here for more information.
To learn more about FSU and TMH review requirements, including administrative or pre-IRB review, visit our Studies involving TMH web page.
*Keep in mind that even when working together, both FSU and TMH have separate federal regulatory responsibilities that must be carefully followed. The administrative or pre-IRB review process is used to determine how those responsibilities will be discharged so that both FSU and TMH are compliant with applicable federal law.
Yes, the IRB will review an agreement that covers studies that may involve human subjects (see footnote 1 therein). For example, the IRB may require that a research agreement has been submitted in the appropriate RAMP module, and that the agreement does or will conform to all applicable regulatory requirements, such as a data use agreement for studies that involve use of certain identifiable health information [link; see footnote 6)], or an IRB reliance agreement for when institutions will defer IRB review to another [link]. In other cases where the IRB may not necessarily require an agreement, the IRB may still review an agreement, such as for example when a term or condition in a clinical trial or sponsored research agreement stipulates that FSU IRB approval is required before research may start or study funds are expended. In all cases where the OHSP/IRB is asked to perform an ancillary review, congruence between the research agreement and the underlying human study will be evaluated.
Except for reliance agreements however, the OHSP and IRB have no signature authority for research agreements (i.e., the OHSP will not actually serve as the FSU signatory/sign the agreement on behalf of FSU). Rather, the OHSP/IRB may be asked by another FSU office to complete an ancillary review and provide its subsequent ancillary review approval for certain agreements to determine whether any terms and conditions related to human subjects protection requirements and IRB approval have been satisfied.
IMPORTANT NOTE: Unless an underlying human study has already been accepted or cleared by the OHSP/IRB or, if already accepted or cleared is consistent with an associated research agreement, an ancillary review made of the OHSP with regard to the agreement will NOT be completed or approved. Therefore, researchers should make certain that: (1) for any research agreement that may be associated with a human study, that the underlying study has been submitted to the FSU IRB using FSU's RAMP IRB system; (2) the underlying study's scope, activities and other parameters are consistent with the research agreement's terms and conditions and any applicable scope of work; and (3) any OHSP request for clarification made of the researcher is timely and thoroughly provided to the OHSP's satisfaction so that the ancillary review may be completed as expeditiously as possible.
See our Research Agreements and Human Studies web page for more complete information, instructions and other resources.
- If you have a specific question not listed above, contact OHSP using the “Add Comment” feature (to the left of the workflow diagram and listed below under Next Steps) on your study workspace to ask your question and select the option to send an email notification about your question to your IRB Coordinator (whose name is listed above and to the left of the workflow); there, in response to the question about who should receive the email notification about your comment, select "IRB Coordinator." This will help to ensure that the IRB Coordinator assigned to your submission will receive a notification that your comment has been posted in your RAMP IRB workspace.

- Alternatively and if you have not yet submitted your study or your specific question is not related to your submitted study, contact OHSP or refer to the contact information in the left panel, and your question will be routed to OHSP staff for follow-up with you directly.
- For FAQs specific to student-led research, visit this student-led research FAQs page.
Need to contact OHSP? Click on the panel below.
Office for Human Subjects Protection (OHSP)
2010 Levy Avenue, Bldg. B Suite 276 (FSU Innovation Park Campus)
Tallahassee FL, 32306-2742 (mailing) or 32310 (physical or courier)
Telephone: (850) 644-7900 (automated call answering with voice menu system allowing callers to be routed quickly and efficiently to needed points of contact)
Facsimile: (850) 644-4392
Email: humansubjects@fsu.edu
Contact information for the IRB and OHSP are in the panel above. The garnet ribbon below is information for the FSU Office of Research or FSU generally, and does not include any information for the IRB or OHSP.
