Information for Medical Students in the FSU College of Medicine
Generally, submission of an IRB application for advance IRB review and approval is required of any College of Medicine (COM) medical student-led activity that may, for a research purpose, involve individuals as respondents or study participants when the activity will include (1) interaction or intervention with individuals to collect their information or biospecimens, or (2) collection or use of individuals' information or biospecimens (e.g., secondary use of previously collected information, even when students will have no interaction or intervention with the individuals). An exception may be made for COM medical student activities conducted solely for purposes of their clerkships; use the "Exceptions" panel below to learn more. In all other cases, COM medical student-led activities may not begin until completion of review and approval or clearance by the FSU Office for Human Subjects Protection (OHSP) and/or Institutional Review Board (IRB); refer to the Submission Requirements panel below.
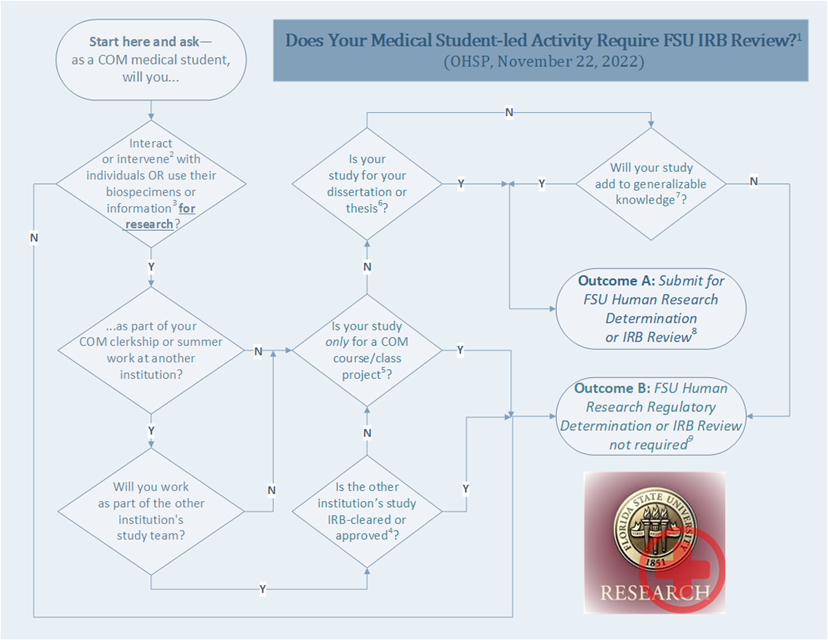
This page is intended to provide FSU COM medical students with instructions, guidance, FAQs and links to templates and other resources for submitting their medical student-led activities for review. The image below depicts an algorithm about when a COM medical student-led activity may require review; click on the image for the complete algorithm, including explanatory notes and instructions.
Click on a panel below to learn more about submission requirements for COM medical student-led research, including exceptions for certain activities that may not require OHSP or IRB review, as well as to access other important information about student-led activities and research.
Contact information for the IRB and OHSP are in the panel above. The garnet ribbon below is information for the FSU Office of Research or FSU generally, and does not include any information for the IRB or OHSP.