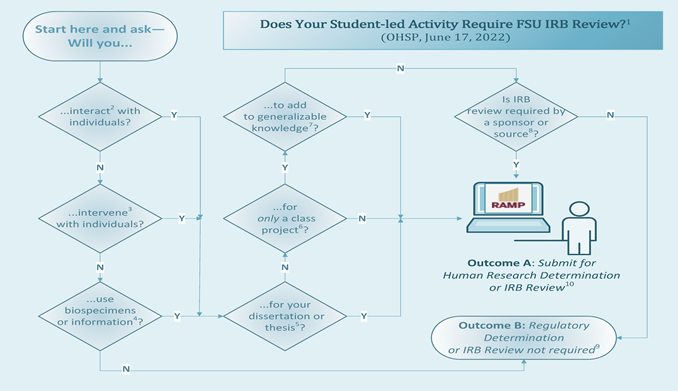
Student-led Activities: Introduction
This page is intended to provide students with instructions, guidance, FAQs and links to templates and other resources for submitting activities for review. The image below depicts an algorithm about when a student-led activity may require IRB review; click on the image for the complete algorithm, including explanatory notes and instructions. If you are a College of Medicine medical student, see our Information for Medical Students in the FSU College of Medicine web page for additional information.
General Submission Requirements
- Any FSU student who plans to undertake activities that may involve individuals who may serve as respondents or study participants or about whom information or biospecimens may be collected or used is required, before initiating or beginning their activity, to have the study submitted for federal regulatory OHSP/IRB review using the FSU Research Administration and Management Portal (RAMP) Institution Review Board (IRB) module (RAMP IRB).
- Paper, email or telephonic submissions or requests for OHSP/IRB review are not accepted.
- See our RAMP IRB video tutorials to see how to use RAMP IRB and navigate a study submission workspace. Tutorials last just a few minutes and include video captures of RAMP IRB workspaces.
- To find out how long it MAY generally take to complete OHSP/IRB review of a student-led activity, refer to FAQ#9, accessible through the Frequently Asked Questions panel below.
VERY IMPORTANT NOTICE TO STUDENTS: FSU dissertation, theses, graduation as well as study sponsor, journal publication, and conference presentation requirements may include the need to provide official documentation of OHSP/IRB approval or clearance (including documentation that further IRB review was not required). Approval or clearance is not granted retroactively (i.e., after or while you are conducting your study). Plan carefully, timely and accordingly so that you have this official documentation.
Need More Information?
Click on one or more of the panels below to obtain additional information, including a list of Frequently Asked Questions, other guidance, and key tips for success.
Contact information for the IRB and OHSP are in the panel above. The garnet ribbon below is information for the FSU Office of Research or FSU generally, and does not include any information for the IRB or OHSP.