Templates & Required Forms
To support the FSU human research community and facilitate their preparation of research-related materials, the IRB and OHSP make available many different templates, including for research protocols and consent-related materials. These templates are designed to standardize the format and organization of content, and help to ensure that the information that is required by applicable federal law for IRB review of research will be included as part of an IRB application.

GENERAL INSTRUCTIONS
When preparing study protocols and consent-related forms, access and use ONLY the FSU approved templates available in RAMP IRB, under the IRB, Library and Templates tabs, as these templates are the most up-to-date and include instructions that are responsive to researchers' questions about the use of these templates and forms. Do not submit for IRB review any materials based upon obsolete templates that you may have used in the past or obtained from colleagues; these may be returned to you for correction. As a guide, below are descriptions about the most frequently used templates and forms.
IMPORTANT NOTE WHEN MODIFYING YOUR FORMS: When modifying or editing your study in order to provide a revision of a previously submitted form such as a protocol or consent form, be sure to use the "Update" feature (the button to the left of a submitted form); do not use "Add" button unless you are submitting an additional form, and do not unless you are replacing a form in its entirety delete the form by using the "x" feature. Revise your form as needed, save the form using the same file name, and after selecting the Update button and in the Edit Attachment window, attach your revised form and add a version number if desired.
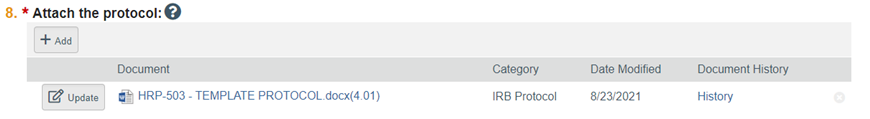
By using the Update feature the RAMP IRB system automatically tracks and pinpoints changes between the updated version and the previous submitted version to facilitate IRB review. Otherwise, the IRB must re-review your form in its entirety rather than just your changes, which will add to the time to complete IRB review.


